Abstract
Steven-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare and sometimes life-threatening hypersensitivity mucocutaneous disease triggered mostly by medication and infections Major involving tissues are the mucous membranes of oral, gastrointestinal, respiratory, integument, and gynecologic tissues. Even after recovering from skin problems without sequelae, survivors can have serious ocular complications leading to blindness despite local and systemic therapy. There is no definite effective systemic and local treatment for SJS/TEN. Early detection and aggressive treatment are important for the long-term prognosis of the eye. Eyelid margin and palpebral conjunctiva and fornix should be checked thoroughly to detect the cicatrical changes that make chronic ocular surface failure such as limbal cell deficiency and complete ocular surface keratinization. Amniotic membrane transplantation and cultivated oral mucosal graft are beneficial to reduce the risk of ocular surface failure.
The ocular surface is mainly composed of conjunctiva and corneal epithelium covered by a thin layer of tear film. Proper eyelid function, lacrimal gland tear production, meibomian gland function and sensorineural factors are essential for homeostasis [1]. Even one dysfunction can make permanent ocular surface problems, which lead to blindness, or ocular surface failure. Ocular surface failure is classified to the two major types; the first is limbal stem cell deficiency (LSCD), in which the corneal epithelium is replaced by conjunctival epithelium and the second is squamous metaplasia, in which the corneal or conjunctival epithelium exhibits keratinization and loss of mucosal epithelial characteristics including the expression of goblet cells. Although there are some options to treat the ocular surface failure, both two types eventually give birth to the dreadful result, blindness.
Steven-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) is a hypersensitivity mucocutaneous disease triggered mostly by medication and infection. They have a direct effect on the skin and no fewer than two mucous membranes including the eye and sometimes can be life-threatening [2]. SJS and TEN are variants belonging in the same class and are defined based upon the amount of epidermal detachment; SJS, 10% or less of total body surface area involvement, TEN, 30% or greater involvement and SJS/TEN overlap, involvement between 10-30% [3]. Clinical findings include a prodromal symptom of fever and malaise, followed by the development of a generalized, tender cutaneous eruption consisting of a variety of morphologic macules, papules, atypical target lesions and vesicles or bullae [4]. Serious dermatologic manifestations may make a physician overlook ocular sequelae, which are irreversible and fatal to visual acuity by the destruction of the ocular surface. The incidence of SJS and TEN are 9.2 and 1.9 per million person-years, respectively [5]. The rareness of SJS/TEN is the obstacle to provide the well-controlled clinical trial and evidence-based treatment. By review of the publications, I focused discussion on the ocular manifestations, prognostic factors and treatments to provide basic understanding to the ophthalmic and the non-ophthalmic physicians.
The pathogenesis of SJS/TEN is controversial. The genetic risk factors are drug-specific and vary among populations and/or ethnic groups. Genetic testing for human leukocyte antigen (HLA)-B*1502 is available and recommended by the U. S. Food and Drug Administration for one drug, carbamazepine, in at-risk (Asian) populations. HLA-B*1502 was strongly associated with carbamazepine-induced SJS/TEN in Han Chinese [6]. Allopurinol is a xanthine oxidase inhibitor commonly prescribed to treat gout and is known to be one of the drugs most frequently associated with SJS and TEN [7]. Allopurinol-induced SJS/TEN has a strong association between HLA-B*58:01 and allopurinol-induced SJS/TEN among Koreans [89]. Genetic associations have been demonstrated for compound medications, such as over-the-counter cold medications [10].
The molecular pathogenesis of SJS/TEN is also not clear. A cytotoxic T lymphocyte (CTL) immune-mediated reaction is known as the major immunologic component of SJS/TEN [1112]. An Immunological memory may cause an early manifestation of recurrent cases of SJS/TEN within 48 hours of repeated provocation. The blister fluid of SJS/TEN patients has shown a predominance of activated CD8+ T lymphocytes, but natural killer (NK) cells and other cytotoxic molecules have also been implicated [13].
Dysregulation of the Fas pathway has been implicated in the pathogenesis of a variety of tissue-destructive processes, including graft-versus-host disease, multiple sclerosis, stroke, and TEN [14]. Viard and colleagues showed that Fas may play a key role in inducing apoptosis in keratinocytes in TEN [15]. They reported that mediation of keratinocyte death in TEN through activation of Fas and by elevated expression of Fas ligand (FasL) on the cell surface of keratinocytes and high levels of soluble sFasL in TEN serum and in frozen skin sections of TEN patients induced apoptosis in a Fas-sensitive cell line, while apoptosis was blocked by anti-FasL-monocolonal antibody. But Chang and colleagues found that sFasL levels peaked 24-48 hours after the onset of significant skin damage, suggesting that sFasL may be a byproduct of FasL expressed on epidermal cells and not a direct inducer of apoptosis [16]. Other immunologic components were suggested such as Gelatinase A (MMP2) and B (MMP9) [17], TNF-R1 and TNF-related apoptosis-inducing ligand (TRAIL) [18], IFN gamma, TNFalpha, sFasL, IL-18, and IL-10 [19] and perforin/granzyme B [20].
The ocular manifestation of SJS/TEN can be changed according to the clinical stages; acute, subacute and chronic (Table 1). The acute stage is usually within 2 weeks after the onset of symptoms. The most common ocular condition observed at this stage is bilateral conjunctivitis, which occurs in 15-75% of patients [2122]. Another 25% of hospitalized patients develop conjunctival and/or corneal ulcerations. A careful lid eversion with fluorescein staining is mandatory to inspect the tarsal and the bulbar conjunctiva.
The pathogenesis of the acute phase of SJS/TEN are the keratinocyte apoptosis and secondary effects of inflammation and loss of ocular surface epithelium. Acute ocular involvement rate is 50% to 88% of SJS/TEN [232425]. Epithelial loss of tarsal conjunctiva and eyelid margin with or without pseudomembrane or true membrane formation can make early symblepharon formation and fornix foreshortening. Corneal epithelial defect can bring out corneal ulceration and perforation [2627]. Meibomianitis has a high prevalence rate, which are more than half of patients [23].
Power and colleagues suggested the schema for the severity of ocular involvement during the acute phase of the disease [24]. To sum up, mild was defined as ocular signs involvement just like lid edema, conjunctival injection, which require prophylactic antibiotics or lubricants and routine eye care, after all completely resolved before discharge from hospital. Moderate involvement was defined as requiring specific treatment. Conjunctival membranes, corneal epithelial loss of more than 30% were typical ocular complications, but vision usually is not affected and the ocular complications were nearly resolved before discharge. Severe involvement meant that the sight-threatening status and the patients needed continuous specific ophthalmic treatment after discharge. Symblepharon showed the active state of corneal disease. Severity was determined by the severity of more severely affected eye.
Even though skin lesions are mostly resolved, chronic cicatrizing conjunctivitis with trichiasis and irregular eyelid margins may be persistent by the inflammation and ulceration of the ocular surface. Lid margin inflammation targets the meibomian glands in particular and causes their widespread destruction, in addition to distichiasis. The abnormal eyelid with misdirected and/or distichiatic lashes a mechanically abrasion on the corneal epithelium, leading to corneal epithelial defects, infection, and stromal scar (Fig. 1). Severe inflammation and persistent ulceration of the tarsal conjunctiva and lid margins leads to lid margin keratinization and tarsal scar [2829].
Sotozono and colleagues developed a grading system for the chronic ocular manifestations in patients with SJS/TEN which is made up by corneal, conjunctival, and eyelid complications and they assessed 13 components and scored them on a scale from 0 to 3 according to their severity [30]. The grading system was based on manifestations observed using slit-lamp biomicroscopy. The classified corneal complications included superficial punctate keratopathy, epithelial defect, loss of the palisades of Vogt, conjunctivalization, neovascularization, opacification, and keratinization. The conjunctival complications included hyperemia and symblepharon formation. Eyelid complications included trichiasis, mucocutaneous junction involvement, meibomian gland involvement, and punctal occlusion.
The chronic ocular sequelae occur in up to 35% of SJS/TEN patients [31]. The chronic stage of the disease is characterized by persistent and prolonged ocular surface inflammation and ulceration. Chronic ocular sequelae with severe visual loss is associated with lid margin abnormality and ocular surface failure [32]. Conjunctival ulcerations or conjunctival membrane formation, or persistent inflammation makes permanent symblepharon and ankyloblepharon, which disrupts a tear film meniscus and inhibits proper eyelid closure and blinking, and sometimes restrict ocular motility [33]. The contracture of the palpebral conjunctiva leads to cicatricial entropion and trichiasis [34]. Tarsal conjunctival scarring can be associated with eyelid malpositions and other disorders, including ectropion, entropion, trichiasis, distichiasis, meibomian gland atrophy and inspissation, punctal occlusion, and keratinization of the eyelid margin, tarsal and bulbar conjunctival surfaces [2833] (Fig. 2). A vicious cycle of ocular surface inflammation and scarring leads to disruption of the delicate architecture and function of the eyelids and tear film, which leads to further progression of the ocular surface damage and increased inflammation.
Chronic ocular sequelae leads to ocular surface failure such as chronic LSCD or squamous metaplasia of ocular surface and is correlated with development of late corneal blindness [28]. Symblepharon and eyelid malposition often worsen over time. Lipid tear deficiency, in which conjunctival cytology showed a marked decrease in goblet cell density [23], and trauma by lid margin abnormality are the main pathogenesis. The keratinized inner eyelid surface can lead directly to chronic corneal inflammation, neovascularization, scarring, and LSCD [2829]. Scarring in the fornices and in the lacrimal gland ducts causes severe aqueous tear deficiency and xerosis [35].
The prevalence of specific ocular abnormalities after SJS/TEN varies widely according to published reports. The lack of standardized criteria for grading the severity of acute ocular involvement may yield variable complication rates across different studies [232836]. The reported mortality rates varied between 1-5% for SJS and 25-35% for patients with TEN. Predictors of mortality included increasing age, increasing number of chronic conditions, infection (septicemia, pneumonia, and tuberculosis), hematological malignancy (non-Hodgkin's lymphoma, leukemia), and renal failure (P≤0.03 for all) [5].
The physician can miss the best opportunity to treat the eye by unawareness of early signs and symptoms of the eyes because they are apt to pay attention to the fatal condition [9]. But, compared with Sjögren syndrome (SS) that is the severe chronic ocular surface inflammatory diseases SJS/TEN is worse in visual acuity, clinical ocular surface score and subjective scores [37]. The acute stage severity or etiology did not correlate to ocular involvement. Generally, ocular complications were related to ocular involvement severity in the acute phase. Early ophthalmic assessment and frequent follow-up are helpful because ocular involvement represents the first long-term complication in patients with TEN [23].
Predictive factors associated with acute ocular involvement in SJS/TEN are young age and the history of taking NSAIDs or cold remedies [38]. This case series suggest that the mortality of children is lower, ranging between 0% and 17%. Antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs are the most commonly implicated etiologies in children [39]. The degree of ocular complication increased the prevalence of visual disturbance and eye dryness [38].
Systemic steroid had no benefit on ocular complications [2440]. The SCORTEN is a severity-of-illness score for SJS and TEN based on a minimal set of well-defined variables derived from the Simplified Acute Physiology Score (SAPS II) that is calculated within 24 hours of admission [2]. In acute stage, the patients with epidermal detachment of more than 10% of the total body surface showed more frequent ocular damage. But the SCORTEN value did not correlate with the severity of eye involvement in the acute setting [41]. The severity of the acute ocular disease and abnormal laboratory tests were not the significant risk factors of late complications [42].
Conjunctivalization of cornea, Cicatricial eyelid and conjunctival complications were correlated with poor vision.
Appropriate early treatment cannot prevent slight cicatrical changes in the eyelid and even slight cicatrical changes can make corneal complications. The lid margin was a commonly affected site.
The goal of treatment of SJS/TEN is the recovery of the systemic condition and prevention of cicatrical ocular complications [43]. Patterns of chronic ocular disease after the acute episode were classified to mild/moderate SJS, severe SJS, ocular surface failure, recurrent episodic inflammation, scleritis and progressive conjunctival cicatrisation resembling mucous membrane pemphigoid [4445]. The severity of ocular involvement was classified as mild, moderate, or severe.
Until now, there was no evidence of therapeutic benefits of systemic immunodulatory therapy in final visual outcome and chronic ocular complications in SJS/TEN [4046]. Ophthalmic treatment depend on the severity and topical eye drops cannot substitute surgical treatment.
In acute SJS/TEN, topical medications for severe conjunctivitis with meibominitis are topical broad-spectrum antibiotic, topical corticosteroid, and a preservative-free lubricant to protect the ocular surface in acute-stage patients. Early topical steroid treatment is important for the improvement of visual prognosis [31]. Amniotic membrane transplantation (AMT) at the acute stage of SJS/TEN with conformer, symblepharon ring or ProKera should cover the all ocular surface [47]. Cryopreserved amniotic membrane was applied over the lid margins, palpebral conjunctiva, and ocular surface, anchored in place with bolstered fornix sutures, perilimbal sutures, and a conformer [324849]. AMT usually performed in the acute stage with the first 2 weeks after the onset of ocular involvement to encourage the rapid epithelial healing and to reduce inflammation and scarring in the ocular surface.
In subacute or chronic stage, the ocular surface can be compromised. Severe dry eye occurs as the result of an abnormality in the tear film components and extensive ocular surface scarring, which lead to a combination of symblepharon formation, limbal stem cell deficiency, and recurrent or persistent corneal epithelial defects [46]. Keratinization of the posterior lid margin and the subsequent repeated microtrauma of the cornea with every blink causes the cornea to become damaged, vascularized, and inflamed. Chronic conjunctival cicatrization leads to the deformation of the lid margins, which causes entropion, trichiasis, and distichiasis. This further exacerbates the damage to the cornea. Di Pascuale and colleagues also reported that the extent of eyelid and tarsal pathology had a significant impact on the occurrence of corneal complications [28]. The treatment strategy during the chronic stage of SJS/TEN revolves around preventing continual ocular surface damage, managing SJS/TEN sequelae, and visual rehabilitation. Although large diameter scleral contact lenses offer substantial benefits to patients with ocular involvement of SJS/TEN, lens fitting can be a problem in eyes with symblepharon [2950]. In addition, patient compliance may be less than optimal after SJS and its high cost is the barrier to its use [29]. The long term use of bandage contact lenses and scleral lenses can lead to the complications, especially in chronic dry eyes [51]. Mucous membrane grafting (MMG) is the efficient treatment to reducing keratinization of palapebral conjunctiva and eyelid margin [4852]. McCord and colleagues reported the earliest clinical series of buccal MMG in SJS and Iyer and colleagues supported the efficacy of MMG in preventing lid margin keratinization [294852].
In the end stage disease, patients have the corneal blindness with severe dry eye. Generally, penetrating keratoplasty should not be performed in the condition of severe dry eye with SJS or TEN because PK does not supply the limbal region of the eye with corneal epithelial stem cells [31324849]. Limbal stem cell transplantation (LSCT) and cultivated oral mucosal epithelial transplantation (COMET) can be the optimal choice to promote the epithelial regeneration with a relatively wet ocular surface [53545556]. In general, patients with SJS/TEN had poorer graft survival rates compared with other LSCD of chemical burn and thermal corneal damage [325556] because these patients present with serious preoperative conditions (e.g., persistent inflammation of the ocular surface, abnormal epithelial differentiation of the ocular surface, severe dry eye and lid-related abnormalities). Additionally, patients with allogenic LSCT inevitably need immunosuppressive treatment and both immunological defenses and the distribution of commensal bacterial on the ocular surface may become modified to some extent, resulting in the occurrence of postoperative bacterial infections, immunological rejection, or sustained ocular surface inflammation [57]. Thus, the long-term prognosis in these patients is poor. In 2002, Nakamura and colleagues first reported ocular surface reconstruction using tissue-engineered autologous oral mucosal epithelial sheets [5354]. Since then, autologous COMET has been used around the world to treat eyes with severe ocular surface disorders, including SJS [58]. A recent summarized report of 242 patients showed that 72% (126/175 eyes) of eyes were classified to successful treatment and 68% (142/210 eyes) of the eyes experienced visual improvement in bilateral LSCD [58]. Naturally, patients who had COMET do not need the immunosuppression, which was different from LSCT.
Keratoprosthesis is replacement of a damaged and opaque cornea with an artificial implant. Clinically used keratoprothetics are the Boston keratoprothesis and Osteo-odonto-keratoprothesis. The Boston keratoprosthesis has a collar button design. It consists of 3 components: a front plate with an optical stem, a back plate, and a titanium locking C-ring [59]. The sandwiched corneal tissue between two plates is used to suture the device to the eye. The Boston Kpro is made of type I and type II formats and the type II is used for severe end stage ocular surface diseases, including Ocular Cicatricial Pemphigoid/SJS. Osteo-odonto-keratoprothesis uses the rooted tooth and the surrounding intact alveolar bone as a plate, which carries a polymethyl methacrylate optical cylinder [606162].
The acute stage may present with bilateral conjunctivitis and loss of the ocular surface epithelium. The chronic stage manifests with cicatricial sequelae and persistent ocular surface inflammation and a chronically dry surface. Although the use of immunosuppressive and immunomodulatory therapies is controversial, the role of AMT in the acute stage is well established and should be performed at the earliest possible opportunity. It is recommended to prevent symblepharon, eyelid malposition, dry eye, and corneal disease rather than to try to reverse the damage later. The chronic stage of SJS is usually characterized by severe dry eye, lid margin abnormalities, and LSCD. Some patients may benefit from systemic immunosuppressive therapy in the chronic stages, but overall, topical and systemic drugs have a limited role to play late in the disease. Most important factors to reduce the chronic ocular surface failure is correcting lid margin deformities and protecting the ocular surface from lid margin keratinization. Oral MMG stabilizes the ocular surface and reduces the damage by the keratinized lid margin.
Figures and Tables
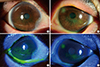
Fig. 1
Subacute/chronic phase Steven-Johnson syndrome. Both eyes show the superficial punctate erosion of more than half of the ocular surface. Lid margins show the irregularity of the mucocutaneous junction and keratin deposition (A, C: right eye, B, D: left eye).
References
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.
2. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000; 115:149–153.

3. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J. CLinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129:92–96.

5. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in united states adults. J Invest Dermatol. 2016.

6. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004; 486.
7. Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008; 58:25–32.

8. Lee HS, Ueta M, Kim MK, Seo KY, Sotozono C, Kinoshita S, et al. Analysis of ocular manifestation and genetic association of allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in South Korea. Cornea. 2016; 35:199–204.

9. Miliszewski MA, Kirchhof MG, Sikora S, Papp A, Dutz JP. Stevens-Johnson syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med. 2016.

10. Ueta M, Kaniwa N, Sotozono C, Tokunaga K, Saito Y, Sawai H, et al. Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe mucosal involvement. Sci Rep. 2014; 4:4862.
11. Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S. Stevens-Johnson syndrome/toxic epidermal necrolysis--a comprehensive review and guide to therapy. I. Systemic Disease. Ocul Surf. 2016; 14:2–19.

12. Friedmann PS, Strickland I, Pirmohamed M, Park B. INvestigation of mechanisms in toxic epidermal necrolysis induced by carbamazepine. Arch Dermatol. 1994; 130:598–604.

13. Saeed HN, Chodosh J. Immunologic mediators in Stevens-Johnson syndrome and toxic epidermal necrolysis. Semin Ophthalmol. 2016; 31:85–90.

14. French LE, Tschopp J. Protein-based therapeutic approaches targeting death receptors. Cell Death Differ. 2003; 10:117–123.

15. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998; 282:490–493.

16. Chang HY, Cooper ZA, Swetter SM, Marinkovich MP. Kinetics and Specificity of fas ligand induction in toxic epidermal necrolysis. Arch Dermatol. 2004; 140:242–244.

17. Gaultier F, Ejeil AL, Igondjo-Tchen S, Dohan D, Dridi SM, Maman L, et al. Possible involvement of gelatinase A (MMP2) and gelatinase B (MMP9) in toxic epidermal necrolysis or Stevens-Johnson syndrome. Arch Dermatol Research. 2004; 296:220–225.

18. Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol. 2005; 153:241–253.

19. Nassif A, Moslehi H, Le Gouvello S, Bagot M, Lyonnet L, Michel L, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004; 123:850–855.

20. Posadas SJ, Padial A, Torres MJ, Mayorga C, Leyva L, Sanchez E, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002; 109:155–161.

22. Howard GM. The Stevens-Johnson syndrome. Ocular prognosis and treatment. Am J Ophthalmol. 1963; 55:893–900.
23. Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano CI, Conesa E, de Juan IE, Murube del Castillo J. Ocular features and histopathologic changes during follow-up of toxic epidermal necrolysis. Ophthalmology. 2011; 118:265–271.

24. Power WJ, Ghoraishi M, Merayo-Lloves J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995; 102:1669–1676.

25. Basu S, Pillai VS, Sangwan VS. Mucosal complications of modified osteo-odonto keratoprosthesis in chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2013; 156:867–873.e2.

26. Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007; 33:1644–1646.

27. Sachdev R, Bansal S, Sinha R, Sharma N, Titiyal JS. Bilateral microbial keratitis in highly active antiretroviral therapy-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a case series. Ocul Immunol Inflamm. 2011; 19:343–345.

28. Di Pascuale MA, Espana EM, Liu DT-S, Kawakita T, Li W, Gao YY, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005; 112:904–912.

29. Iyer G, Pillai VS, Srinivasan B, Guruswami S, Padmanabhan P. Mucous membrane grafting for lid margin keratinization in Stevens-Johnson syndrome: results. Cornea. 2010; 29:146–151.

30. Sotozono C, Ang LP, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007; 114:1294–1302.

31. Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, Ikezawa Z, et al. Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology. 2009; 116:685–690.

32. Jain R, Sharma N, Basu S, Iyer G, Ueta M, Sotozono C, et al. Stevens-Johnson syndrome: the role of an ophthalmologist. Surv Ophthalmol. 2016.

33. Tseng SC, Di Pascuale MA, Liu DT, Gao YY, Baradaran-Rafii A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology. 2005; 112:896–903.

34. Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009; 54:686–696.

35. Wall V, Yen MT, Yang MC, Huang AJ, Pflugfelder SC. Management of the late ocular sequelae of Stevens-Johnson syndrome. Ocul Surf. 2003; 1:192–201.

36. Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea. 2007; 26:123–129.

37. Kaido M, Yamada M, Sotozono C, Kinoshita S, Shimazaki J, Tagawa Y, et al. The relation between visual performance and clinical ocular manifestations in Stevens-Johnson syndrome. Am J Ophthalmol. 2012; 154:499–511.e1.

38. Sotozono C, Ueta M, Nakatani E, Kitami A, Watanabe H, Sueki H, et al. Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2015; 160:228–237.e2.

39. Catt CJ, Hamilton GM, Fish J, Mireskandari K, Ali A. Ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Am J Ophthalmol. 2016.
40. Kim DH, Yoon KC, Seo KY, Lee HS, Yoon SC, Sotozono C, et al. The role of systemic immunomodulatory treatment and prognostic factors on chronic ocular complications in Stevens-Johnson syndrome. Ophthalmology. 2015; 122:254–264.

41. Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. 2010; 150:505–510.e1.

42. Yip LW, Thong BY, Lim J, Tan AW, Wong HB, Handa S, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy. 2007; 62:527–531.

43. Saeed H, Mantagos IS, Chodosh J. Complications of Stevens-Johnson syndrome beyond the eye and skin. Burns. 2016; 42:20–27.

44. De Rojas MV, Dart JK, Saw VP. The natural history of Stevens Johnson syndrome: patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br J Ophthalmol. 2007; 91:1048–1053.

45. Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015; 16:475–493.

46. Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y, Nakamura K, Kambara T, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients--Treatment and outcome. Allergol Int. 2016; 65:74–81.

47. Shay E, Khadem JJ, Tseng SC. Efficacy and limitation of sutureless amniotic membrane transplantation for acute toxic epidermal necrolysis. Cornea. 2010; 29:359–361.

48. Iyer G, Srinivasan B, Agarwal S, Pillai VS, Ahuja A. Treatment modalities and clinical outcomes in ocular sequelae of Stevens-Johnson syndrome over 25 years--a paradigm shift. Cornea. 2016; 35:46–50.

49. Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic Disease. Ocul Surf. 2016; 14:168–188.

50. Weber SL, de Souza RB, Gomes JA, Hofling-Lima AL. The use of the esclera scleral contact lens in the treatment of moderate to severe dry eye disease. Am J Ophthalmol. 2016; 163:167–173.e1.
51. Sindt CW, Longmuir RA. Contact lens strategies for the patient with dry eye. Ocul Surf. 2007; 5:294–307.

52. McCord CD Jr, Chen WP. Tarsal polishing and mucous membrane grafting for cicatricial entropion, trichiasis and epidermalization. Ophthalmic Surg. 1983; 14:1021–1025.

53. Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004; 88:1280–1284.

54. Nakamura T, Koizumi N, Tsuzuki M, Inoki K, Sano Y, Sotozono C, et al. Successful regrafting of cultivated corneal epithelium using amniotic membrane as a carrier in severe ocular surface disease. Cornea. 2003; 22:70–71.

55. Shimazaki J, Maruyama F, Shimmura S, Fujishima H, Tsubota K. Immunologic rejection of the central graft after limbal allograft transplantation combined with penetrating keratoplasty. Cornea. 2001; 20:149–152.

56. Tseng SG, Prabhasawat P, Barton K, Gray T, Meller D. AMniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116:431–441.

57. Venugopal R, Satpathy G, Sangwan S, Kapil A, Aron N, Agarwal T, et al. Conjunctival microbial flora in ocular Stevens-Johnson syndrome sequelae patients at a tertiary eye care center. Cornea. 2016.

58. Utheim TP. Concise review: transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency—current status and future perspectives. Stem Cells. 2015; 33:1685–1695.

59. Sayegh RR, Ang LPK, Foster CS, Dohlman CH. The boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol. 2008; 145:438–444.

60. Strampelli B, Valvo A, Tusa E. Osteo-odonto-keratoprosthesis in a case treated for anchylobepharon and total simbleraphon. Annali di Ottalmologia e Clinica Oculistica. 1965; 91:462–479.
61. Strampelli B. Technical improvements in osteo-odonto-keratoprothesis. Annali di Ottalmologia e Clinica Oculistica. 1966; 92:155–178.
62. Strampelli B. Osteo-chondro-keratoprosthesis in substitution of the osteo-odonto-keratoprosthesis in edentulous patients. Annali di Ottalmologia e Clinica Oculistica. 1967; 93:975–978.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download