Abstract
Proximal pulmonary artery aneurysms and dissection are rare and life-threatening conditions, which are usually detected only during autopsy examination in cases of sudden death. These pathological entities often occur as complications of chronic pulmonary hypertension and most commonly result from pulmonary arterial hypertension associated with various congenital cardiac lesions involving left-to-right shunting. This study describes an autopsy case of a 38-year-old man who was diagnosed with Eisenmenger syndrome 5 years prior to sudden death secondary to cardiac tamponade following a ruptured pulmonary trunk aneurysm.
Proximal pulmonary artery aneurysms and dissection are rare and life-threatening conditions [12345678910] that often lead to sudden death secondary to hemorrhagic cardiac tamponade caused by a ruptured proximal pulmonary artery aneurysm and may be identified only during autopsy examination. Although various etiopathogenetic contributors have been identified, usually these conditions occur as a complication of chronic pulmonary hypertension. Pulmonary hypertension is classically categorized into primary and secondary types; proximal pulmonary artery aneurysms and dissection are commonly associated with secondary pulmonary hypertension in a setting of congenital cardiac lesions in infants with various forms of left-to-right shunts [11]. Eisenmenger syndrome is characterized by elevated pulmonary vascular resistance and occurs as a complication of uncorrected congenital heart anomalies that cause left-to-right shunts [12]. Increased pulmonary resistance often develops over time and reverses left-to-right shunting. Mortality associated with Eisenmenger syndrome is attributable to two distinct causes-progressive heart failure and sudden death [13]. Life expectancy of patients with Eisenmenger syndrome depends on the type and severity of the underlying congenital anomaly and ranges from 20 to 50 years [12].
This report describes an autopsy case of a 38-year-old man who was diagnosed with Eisenmenger syndrome 5 years prior to sudden death secondary to cardiac tamponade following a ruptured pulmonary trunk aneurysm.
A 38-year-old man with a history of dyspnea was diagnosed with Eisenmenger syndrome secondary to a large ventricular septal defect 5 years prior to sudden death. Surgical correction of the cardiac defect was deemed unfeasible. He was subsequently treated with loop diuretics, angiotensin-converting enzyme inhibitors, and calcium channel blockers. He was discovered dead, kneeling next to his bed with his chin on the bed. Several purple spots were observed on the anterior surface of his body without any evidence of trauma.
Autopsy findings revealed that the pericardial sac was filled with approximately 1,250 mL of blood. The pulmonary trunk showed aneurysmal dilatation (circumferential size 17 mm), originating 20 mm distal to the orifice of the pulmonary valve and extending 50 mm toward the pulmonary artery bifurcation (Fig. 1). Dissection and aneurysmal rupture occurred through a 50 mm long transverse tear in the wall of the aneurysm. The hemorrhage was attributed to the ruptured pulmonary trunk aneurysm. The pulmonary trunk showed minimal diffuse fatty streaks and yellowish plaques on the intimal surface (Fig. 2). Thrombi were identified in the peripheral small pulmonary arteries of the lung parenchyma and in the proximal large pulmonary arteries including the right upper pulmonary artery, the left upper and lower pulmonary arteries, and the aneurysmal site (Fig. 3). The lungs showed no other gross abnormalities except small organized thrombi. The heart (weight 1,080 g) revealed a large perimembranous ventricular septal defect measuring 25 mm in maximal diameter, right ventricular hypertrophy (maximum wall thickness of 0.9 cm) with dilatation, and concentric left ventricular hypertrophy (maximum wall thickness of 1.2 cm) (Fig. 4). The coronary arteries showed no atherosclerotic stenosis. The other organs showed no specific gross abnormalities.
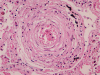
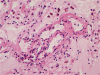
Histopathological examination of the dissected wall of the pulmonary trunk aneurysm showed true and false lumina occupied by organized thrombi. Intimal fibrosis and a focal decrease in the density of elastic fibers were observed in the non-dissected regions of the aneurysmal wall. Destruction and fragmentation of elastic fibers and fibrosis in the adventitia and media were evident within the vascular wall at the dissection site. The lungs revealed findings consistent with severe pulmonary hypertension along with extensive muscular hypertrophy and intimal fibrosis in the pulmonary arteries. Concentric laminar intimal fibrosis, thrombi, intravascular fibrous septa secondary to recanalization, plexiform lesions, and muscularization of arterioles were observed (Figs. 5, 6). Other vascular changes such as fibrinoid necrosis were not observed. Disarrayed hypertrophic cardiac myocytes and interstitial fibrosis were observed on microscopic examination of sections of both, the right and the left ventricular walls.
Pulmonary artery aneurysms are a rare vascular anomaly [1234567891011]. Deterling and Clagett reported an incidence of only 8 cases among 109,571 autopsy cases [2]. Reportedly, pulmonary artery aneurysms usually occur along the branches of the main right or left pulmonary artery [2]. However, in the patient described in this report, the aneurysm affected the pulmonary trunk. Clinically, pulmonary artery aneurysms are associated with dissection, rupture, and often fatal pericardial or mediastinal hemorrhage, thromboembolism, and a mass effect causing compression of adjacent structures.
Pulmonary artery dissection invariably occurs in the area of a pulmonary artery aneurysm and shows a highly variable and nonspecific clinical presentation including dyspnea, syncope, and chest pain, among other such manifestations. This type of dissection is commonly life-threatening and is often associated with sudden death because it precipitates hemodynamic instability or hemorrhagic cardiac tamponade secondary to aneurysm rupture. Pulmonary artery dissection can affect individuals of any age (26 days to 85 years) [25]. A slight female predominance (male:female ratio 1:1.2) and a bimodal incidence peak are observed in the third and sixth decades of life.
Pulmonary artery aneurysms are associated with weakening of the vessel wall and occur most commonly as a complication of chronic pulmonary hypertension, which is defined clinically as a mean pulmonary arterial pressure >25 mm Hg at rest, resulting from congenital or acquired cardiovascular disorders [2711]. Other less common etiologies include connective tissue disorders, infection, trauma, atherosclerosis, pregnancy, amyloidosis, fibromuscular dysplasia, and Behcet's disease [27]. These disorders can be categorized into those involving mechanisms that increase wall stress and those involving mechanisms that alter the strength of the medial wall of the pulmonary artery. Increased pressure in the systemic or pulmonary vasculature increases wall stress. This positive correlation between wall stress and pressure is described by Laplace's Law, which states that wall stress is directly proportional to the pressure and the radius of the vessel wall and inversely proportional to wall thickness. An important trigger for pulmonary hypertension is pulmonary blood overflow, which usually occurs in various cardiac anomalies involving left-to-right shunts, such as ventricular and atrial septal defects and patent ductus arteriosus. In the present case, it was strongly suspected that the large congenital ventricular septal defect with a left-to-right shunt resulted in sustained high pulmonary blood flow and pulmonary arterial hypertension. Increased wall stress secondary to pulmonary arterial hypertension consequently triggered pulmonary artery aneurysm formation, dissection, and rupture. Histopathologically, the aneurysmal wall in the deceased patient revealed findings consistent with medial degeneration indicating weakness of the vessel wall. It has been shown that this medial degeneration can be induced and exacerbated by increased hemodynamic stresses.
Heath and Edwards proposed a 6-tiered grading system for pulmonary hypertension [11], which is based on vascular changes and is useful to assess the severity of pulmonary arterial hypertension. In the present case, microscopic examination of sections of the lung showed medial hypertrophy, intimal proliferation and marked intimal fibrosis, concentric laminar intimal fibrosis, organized and recanalized thrombi, and plexiform lesions in the pulmonary artery. These findings correspond to Heath and Edwards grade IV disease or irreversible severe hypertension. Small thrombi and recanalized thrombi were identified in this patient. Pulmonary thromboembolism is most commonly associated with venous emboli originating from deep leg vein thrombi above the level of the knee. However, there was no evidence of deep vein thrombosis in the deceased. Previous studies have reported that pulmonary artery thrombosis commonly occurs in patients with Eisenmenger syndrome [14]. Unfortunately, the exact pathophysiology remains unclear; therefore, confirming the effectiveness of and establishing guidelines for anticoagulant therapy in such patients has not been possible.
Thrombus formation in pulmonary artery aneurysms is attributed to the following pathophysiological mechanism: sustained wall stress induced by high pulmonary artery pressure (deduced from Laplace's Law), causes vascular wall injury and dilatation, confirmed by histopathological findings such as medial hypertrophy and intimal proliferation in the aneurysmal wall. Vessel wall dilatation consequently causes turbulence or low blood flow velocity (stasis). Hemodynamic changes (turbulence or stasis) constitute one of the three primary factors of the Virchow's triad [15] that contributes to thrombosis, the other two being endothelial injury and hypercoagulability. Thus, it can be concluded that both, mechanical vascular wall injury and alterations in blood flow contribute to thrombogenesis in patients with Eisenmenger syndrome.
In our view, detailed clinical examination and regular follow-up examinations including computed tomography could have diagnosed the pulmonary artery aneurysm prior to the occurrence of the pulmonary artery dissection and rupture and perhaps prevented sudden death in this patient. Despite the rarity of these entitites, pulmonary artery aneurysms and dissection are invariably life-threatening conditions. Therefore, clinicians should consider these among the possible complications during follow-up in patients with pulmonary artery hypertension. Thrombosis should also be considered as an important complication in patients with pulmonary artery hypertension associated with congenital cardiac anomalies. The findings of this report and review of the contemporary literature would provide a better understanding of the histopathological features of this condition and lead to greater awareness among clinicians regarding the possibility of this life-threatening complication in patients with Eisenmenger syndrome.
Figures and Tables
Fig. 1
The pulmonary trunk shows aneurysmal dilatation (arrowheads) with thrombus and is dissected circumferentially (arrows).
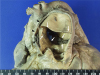
Fig. 2
The right pulmonary artery shows fatty streaks and atheromas on the intimal surface along its branches.
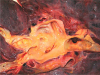
Fig. 3
The lung parenchyma shows two organizing thrombi (arrows) in the branches of the pulmonary artery.
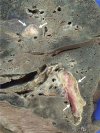
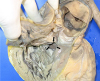
Fig. 4
A large perimembranous ventricular septal defect (arrows) measuring 25 mm at its maximal diameter is seen below the pulmonary valves in the right ventricle.
References
1. Khattar RS, Fox DJ, Alty JE, et al. Pulmonary artery dissection: an emerging cardiovascular complication in surviving patients with chronic pulmonary hypertension. Heart. 2005; 91:142–145.

2. Graham JK, Shehata B. Sudden death due to dissecting pulmonary artery aneurysm: a case report and review of the literature. Am J Forensic Med Pathol. 2007; 28:342–344.
3. Senbaklavaci O, Kaneko Y, Bartunek A, et al. Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment: review and case report. J Thorac Cardiovasc Surg. 2001; 121:1006–1008.
4. Butto F, Lucas RV Jr, Edwards JE. Pulmonary arterial aneurysm: a pathologic study of five cases. Chest. 1987; 91:237–241.
5. Jones SL. Dissecting hematomas of the pulmonary artery: rare and fatal catastrophies. Am J Forensic Med Pathol. 1997; 18:349–353.
6. Walley VM, Virmani R, Silver MD. Pulmonary arterial dissections and ruptures: to be considered in patients with pulmonary arterial hypertension presenting with cardiogenic shock or sudden death. Pathology. 1990; 22:1–4.

7. Inayama Y, Nakatani Y, Kitamura H. Pulmonary artery dissection in patients without underlying pulmonary hypertension. Histopathology. 2001; 38:435–442.

8. Lopez-Candales A, Kleiger RE, Aleman-Gomez J, et al. Pulmonary artery aneurysm: review and case report. Clin Cardiol. 1995; 18:738–740.

9. Arena V, De Giorgio F, Abbate A, et al. Fatal pulmonary arterial dissection and sudden death as initial manifestation of primary pulmonary hypertension: a case report. Cardiovasc Pathol. 2004; 13:230–232.
10. Sakuma M, Demachi J, Suzuki J, et al. Proximal pulmonary artery aneurysms in patients with pulmonary artery hypertension: complicated cases. Intern Med. 2007; 46:1789–1793.

11. Katzenstein AL. Katzenstein and Askin's surgical pathology of non-neoplastic lung disease. Volume 13 in the major problems in pathology series. 4th ed. Philadelphia: Elsevier Saunders;2006. p. 351–384.
12. Vongpatanasin W, Brickner ME, Hillis LD, et al. The Eisenmenger syndrome in adults. Ann Intern Med. 1998; 128:745–755.

13. Sreeram N. Eisenmenger syndrome: towards identifying the risk factors for death. Eur Heart J. 2006; 27:1644–1645.

14. Broberg CS, Ujita M, Prasad S, et al. Pulmonary arterial thrombosis in eisenmenger syndrome is associated with biventricular dysfunction and decreased pulmonary flow velocity. J Am Coll Cardiol. 2007; 50:634–642.

15. Kumar V, Abbas AK, Fausto N. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders;2004. p. 119–144.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download