Abstract
Dural arteriovenous fistula (D-AVF) at the foramen magnum is an extremely rare disease entity. It produces venous hypertension, and can lead to progressive cervical myelopathy thereafter. On the other hand, the venous hypertension may lead to formation of a venous varix, and it can rarely result in an abrupt onset of subarachnoid hemorrhage (SAH) when the venous varix is ruptured. The diagnosis of D-AVF at the foramen magnum as a cause of SAH may be difficult due to its low incidence. Furthermore, when the D-AVF is fed solely by the ascending pharyngeal artery (APA), it may be missed if the external carotid angiography is not performed. The outcome could be fatal if the fistula is unrecognized. Herein, we report on a rare case of SAH caused by ruptured venous varix due to D-AVF at the foramen magnum fed solely by the APA. A review of relevant literatures is provided, and the treatment modalities and outcomes are also discussed.
Dural arteriovenous fistula (D-AVF) at the foramen magnum is an extremely rare disease entity. It arterializes nearby venous systems and produces venous hypertension (HTN). According to previous articles, the patients with D-AVF usually develop progressive clinical symptoms such as cervical myelopathy, radiculopathy, tinnitus, cranial nerve palsy, and posterior neck pain. Meanwhile, the venous HTN caused by D-AVF may also lead to formation of a venous varix, and the patients may suffer from an abrupt onset of a subarachnoid hemorrhage (SAH) when the varix is ruptured, although it is extremely rare.1)3)8)18) The diagnosis of D-AVF at the foramen magnum as a cause of SAH may be difficult due to its low incidence, and the outcome could be fatal if the fistula is unrecognized. Especially when the D-AVF is fed solely by the ascending pharyngeal artery (APA), the lesion cannot be detected if the external carotid angiography is not performed, and it could be misdiagnosed as a non-aneurysm SAH which often follows a benign course. Besides, accurate analysis of feeding arteries and drainage patterns is important for planning the therapeutic strategy because it demands a meticulous caution in process manipulating such a dangerous feeding artery as the APA.2)20) Herein, we report on a rare case of SAH caused by ruptured venous varix due to D-AVF at the foramen magnum fed solely by the APA. A review of relevant literatures is provided, and the treatment modalities and outcomes are also discussed.
A 48-year-old man experienced a sudden severe headache and neck pain while stretching his neck, and had another similar pain during defecation ten days later. After three more days, he visited emergency room with far advanced symptoms. He was in a slightly deteriorated mentality and complained a severe headache, but did not have any motor or sensory deficits. Brain computerized tomography (CT) showed SAH at the cistern magna and focally on the left side of the foramen magnum (Fig. 1). Cerebral angiography revealed a D-AVF at the foramen magnum fed by the hypoglossal branch of the neuromeningeal trunk of left APA and drained into the suboccipital venous plexus and sigmoid sinus (Fig. 2). There was a venous varix adjacent to the fistulous point, and it matched to the location of hematoma.
An optimal working angle at which the origin of the APA, the course of the feeding artery, and the configuration of the target are best demonstrated is determined (Fig. 3). Superselective angiography via the hypoglossal branch of the neuromeningeal trunk of the APA was performed to delineate the anatomical locations of the fistulous point and the ruptured venous varix. A microcatheter was navigated to the point of fistula. After injection of dimethyl sulfoxide for filling of the inner lumen of the microcatheter, Onyx was slowly injected. Overall 0.4 mL of Onyx was used to completely obliterate the D-AVF and venous varix (Fig. 4). There were no major refluxes into the main branch of APA.
His post-procedural course was uneventful. His symptom gradually disappeared within a few days. The follow-up angiography after 1 month also showed complete occlusion of the lesions. He discharged without any neurological deficits.
D-AVF at the foramen magnum accounts for only an extreme minor proportion of D-AVFs in the whole neurovascular system, and relevant reports are insufficient. Cases of D-AVF at this location fed solely by the APA presenting with SAH due to ruptured venous varix are even more rare and unique. Although reports with large series of D-AVF confined to the foramen magnum are absent, there have been a couple of review articles on D-AVF at the craniocervical junction analyzing the unique characteristics of this lesion. Zhao et al.24) reviewed 56 previously reported cases of D-AVFs at the craniocervical junction, and the result showed male predominance with mean age of 55.6 years. Twenty-one patients (37.5%) of them presented with SAH, and others with myelopathy, radiculopathy, cranial nerve palsy, brainstem or cerebellar dysfunction, and occipital neuralgia. Almost all of those D-AVFs were fed by branches of the vertebral artery, exclusively or in combination with the occipital artery or ascending pharyngeal artery. There were only three cases fed solely by the APA in this series, and they were all located at the foramen magnum. The authors suggested that presence of intracranial venous drainage and venous varix was associated with an increased risk of SAH. Superiorly directing drainage into the intracranial venous systems, including cerebral sinuses and cortical veins, has relatively faster venous flow than inferiorly directing drainage into the venous systems of the spinal cord, and increased hemodynamic stress may lead to varix formation and result in SAH.23)24) Aviv et al.2) reviewed 44 cases; 20 patients (45.5%) with SAH and remaining with myelopathy, radiculopathy, tinnitus, and cranial nerve palsy. The authors also showed consistent risk factors associated with bleeding. Spinal D-AVFs most commonly occur at the dorsal thoracolumbar junction. However, the craniocervical D-AVFs differ from the thoracolumbar D-AVFs in terms of clinical pictures and produce more diverse symptoms including higher risk of bleeding.
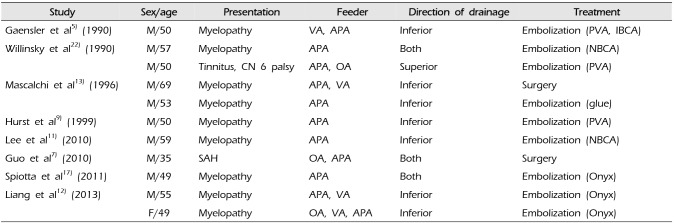
D-AVF at the foramen magnum is a subset of craniocervical D-AVF with the fistulous point at the foramen magnum. It seems to have similar features as any other craniocervical D-AVFs because majority of D-AVFs at the foramen magnum are also fed by the branches of the vertebral artery and drain in similar pattern.7)15) The different point in our case is that the fistula is fed solely by the APA. In order to analyze the clinical features of this specific D-AVF at the foramen magnum fed by the APA, we searched relevant published articles, and overall eleven corresponding cases were found in eight articles (Table 1).5)7)9)11)12)13)17)22) Nine patients presented with myelopathy, only one with SAH, and remaining one with pulsatile tinnitus and abducent nerve palsy. Seven patients had only inferior directing drainage into the spinal venous systems and they all presented with myelopathic symptoms. One patient had only superior directing drainage into the intracranial venous systems and suffered from tinnitus and cranial nerve palsy. Remaining three patients (one presenting with SAH, two with myelopathy) had both superior and inferior drainages as in our case. Similar to the craniocervical D-AVF, predominance of the superior venous drainage seems to produce intracranial symptoms and predominance of the inferior venous drainage seems to produce spinal symptoms. Five patients, or overall six including ours, had the fistula at the foramen magnum fed solely by the APA, and our case was the only one presenting with SAH (approximately 16.7%). The incidence of bleeding seems to be low in the D-AVF at the foramen magnum fed solely by the APA.
When dealing with patients with SAH, diagnosis of D-AVF as a cause of SAH is considerably challenging because SAH due to ruptured intracranial aneurysm accounts for more than 75% of nontraumatic SAH and SAH due to D-AVF at the foramen magnum is extremely rare.10) Although brain CT and magnetic resonance image may provide some clues to localize the bleeding focus and to confirm the presence of abnormal of vascular structure, cerebral angiography still remains to be the gold standard diagnostic tool for detailed evaluation of the vascular anatomy in relation with the feeding artery, fistula, and draining vein. However, it has been reported that the cerebral angiography may fail to identify the lesion for various reasons such as spontaneous obliteration, thrombosis, mass effect, compression, or inadequate study.2)24) According to the report by Aviv et al.2), the initial angiography failed to show the lesion in seven (35%) of 20 patients with SAH caused by craniocervical D-AVF. The authors emphasized importance of performing right vertebral angiography because approximately 63% of craniocervical D-AVF arose from the right vertebral artery and six of the seven patients in whom the initial angiography showed negative results later showed the fistula supplied from the right vertebral artery. Furthermore, for diagnosis of the D-AVF fed solely by the APA as in our case, the external carotid angiography is necessary. Therefore, in order to prevent misdiagnosis, a complete set of cerebral angiography including bilateral internal and external carotid arteries, vertebral arteries, thyrocervical and costocervical trunks plus superselective angiography for the identified feeding arteries are required for adequate diagnosis and evaluation of D-AVF at the foramen magnum.2)4)24)
For a successful and safe endovascular embolization of D-AVF fed by the APA, detailed inspection of superselective angiogram and precise recognition of anatomical configuration are mandatory, because the APA is notorious for dangerous anastomoses to the carotid and vertebral arteries as well as vital supply to the cranial nerves 9, 10, 11, and 12 via the neuromeningeal trunk.1)6)8)10)19)21) Although embolization of D-AVF using polyvinyl alcohol particles, isobutyl 2-cyanoacrylate, and n-butyl cyanoacrylate has been previously described, it is well known that these materials are often related with incomplete occlusion and recanalization after the treatment due to the poor distal penetration and inadequate delivery of the embolisates.24) Recently, Onyx has been increasingly used to treat such lesions because it enables prolonged and controlled injection with enhanced penetration to the target.24) In order to minimize the risk of misembolization of non-target tissue, the tip of the microcatheter should be advanced as close to the fistulous point as possible and also should be located distal to the origin of nontarget branches.12) Slow and steady injection with detailed fluoroscopic inspection is required to prevent major reflux into the main artery. If the microcatheter cannot be navigated close enough to the target lesion or no reflux is allowed due to adjacent vital branch, balloon-augmented Onyx embolization could be an option to improve the penetration of Onyx to the distant target and to prevent major reflux reliably.6)12)14)16)17)24)
D-AVF at the foramen magnum, although extremely rare, should be considered as a possible cause of SAH. Detailed inspection of a complete set of cerebral angiography is mandatory to prevent misdiagnosis. Onyx embolization can be a feasible treatment option to treat such D-AVF. When the D-AVF is fed by the neuromeningeal trunk of APA, precise recognition of anatomical location of the lesion by superselective angiography and accurate delivery of embolic materials to the fistulous point are required for a successful and safe embolization, because there exist dangerous anastomoses between the APA and carotid and vertebral arteries as well as vital supply to the cranial nerves and it carries a significant risk.
References
1. Ansari SA, Lassig JP, Nicol E, Thompson BG, Gemmete JJ, Gandhi D. Transarterial embolization of a cervical dural arteriovenous fistula. Presenting with subarachnoid hemorrhage. Interv Neuroradiol. 2006; 12. 12(4):313–318. PMID: 20569588.
2. Aviv RI, Shad A, Tomlinson G, Niemann D, Teddy PJ, Molyneux AJ, et al. Cervical dural arteriovenous fistula manifesting as subarachnoid hemorrhage: report of two cases and literature review. AJNR Am J Neuroradiol. 2004; 5. 25(5):854–858. PMID: 15140735.
3. Cavalcanti DD, Reis CV, Hanel R, Abbasi SS, Deshmukh P, Spetzler RF, et al. The ascending pharyngeal artery and its relevance for neurosurgical and endovascular procedures. Neurosurgery. 2009; 12. 65(6 Suppl):114–120. discussion 120. PMID: 19934985.

4. Do HM, Jensen ME, Cloft HJ, Kallmes DF, Dion JE. Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol. 1999; 2. 20(2):348–350. PMID: 10094368.
5. Gaensler EH, Jackson DE Jr, Halbach VV. Arteriovenous fistulas of the cervicomedullary junction as a cause of myelopathy: radiographic findings in two cases. AJNR Am J Neuroradiol. 1990; 5. 11(3):518–521. PMID: 2112318.
6. Gross BA, Albuquerque FC, Moon K, Mcdougall CG. The road less traveled: transarterial embolization of dural arteriovenous fistulas via the ascending pharyngeal artery. J NeuroInterv Surg. 2017; 1. 9(1):97–101. PMID: 27581042.

7. Guo LM, Zhou HY, Xu JW, Wang GS, Tian X, Wang Y, et al. Dural arteriovenous fistula at the foramen magnum presenting with subarachnoid hemorrhage: case reports and literature review. Eur J Neurol. 2010; 5. 17(5):684–691. PMID: 20050886.

8. Hacein-Bey L, Daniels DL, Ulmer JL, Mark LP, Smith MM, Strottmann JM, et al. The ascending pharyngeal artery: branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol. 2002; 8. 23(7):1246–1256. PMID: 12169487.
9. Hurst RW, Bagley LJ, Scanlon M, Flamm ES. Dural arteriovenous fistulas of the craniocervical junction. Skull Base Surg. 1999; 9(1):1–7.

10. Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004; 4. 100(4 Suppl Spine):385–391. PMID: 15070151.
11. Lee JY, Cho YD, Kwon BJ, Han MH. Dural arteriovenous fistula at the foramen magnum with holocord myelopathy: case report. Neurointervention. 2010; 2. 5(1):53–57.

12. Liang G, Gao X, Li Z, Wang X, Zhang H, Wu Z. Endovascular treatment for dural arteriovenous fistula at the foramen magnum: report of five consecutive patients and experience with balloon-augmented transarterial Onyx injection. J Neuroradiol. 2013; 5. 40(2):134–139. PMID: 23433906.

13. Mascalchi M, Scazzeri F, Prosetti D, Ferrito G, Salvi F, Quilici N. Dural arteriovenous fistula at the craniocervical junction with perimedullary venous drainage. AJNR Am J Neuroradiol. 1996; Jun-Jul. 17(6):1137–1141. PMID: 8791928.
14. Mondel PK, Saraf R, Limaye US. Acute subarachnoid hemorrhage in posterior condylar canal dural arteriovenous fistula: imaging features with endovascular management. J Neurointerv Surg. 2015; 7. 7(7):e26. PMID: 25006042.

15. Reinges MHT, Thron A, Mull M, Huffmann BC, Gilsbach JM. Dural arteriovenous fistulae at the foramen magnum. J Neurol. 2001; 3. 248(3):197–203. PMID: 11355153.

16. Sadeh-Gonik U, Gory B, Riva R, Labeyrie PE, Signorelli F, Lukaszewicz AC, et al. Ethylene vinyl alcohol copolymer (Onyx®) embolization of cranial dural arteriovenous fistula via the ascending pharyngeal artery. Diagn Interv Imaging. 2016; 6. 97(6):681–685. PMID: 26867991.
17. Spiotta AM, Hughes G, Masaryk TJ, Hui FK. Balloon-augmented Onyx embolization of a dural arteriovenous fistula arising from the neuromeningeal trunk of the ascending pharyngeal artery: technical report. J NeuroInterv Surg. 2011; 9. 3(3):300–303. PMID: 21990848.

18. Suda S, Katsura KI, Okubo S, Abe A, Kanamaru T, Ueda M, et al. A case of dural arteriovenous fistulas at the craniocervical junction presenting with occipital/neck pain associated with sleep. Intern Med. 2012; 4. 51(8):925–928. PMID: 22504252.

19. Sun HS, Yun HS, Song MK, Han JY, Choi IS, Lee SG. Dural arteriovenous fistula on the brain stem and upper cervical spinal cord - a case report -. Ann Rehabil Med. 2011; 10. 35(5):733–737. PMID: 22506199.

20. Tanoue S, Goto K, Oota S. Endovascular treatment for dural arteriovenous fistula of the anterior condylar vein with unusual venous drainage: report of two cases. AJNR Am J Neuroradiol. 2005; 9. 26(8):1955–1959. PMID: 16155141.
21. Viñuela F, Fox AJ, Pelz DM, Drake CG. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg. 1986; 4. 64(4):554–558. PMID: 3950738.

22. Willinsky R, Terbrugge K, Lasjaunias P, Montanera W. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol. 1990; 8. 34(2):118–123. PMID: 2367931.

23. Wu Q, Wang HD, Shin YS, Zhang X. Brainstem congestion due to dural arteriovenous fistula at the craniocervical junction. J Korean Neurosurg Soc. 2014; 3. 55(3):152–155. PMID: 24851151.
24. Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction : a systematic review. J NeuroInterv Surg. 2016; 6. 8(6):648–653. PMID: 26041099.
Fig. 1
Initial brain computerized tomography shows subarachnoid hemorrhage at the cistern magna (A). Focal hematoma (white arrow) is observed on the left side of the foramen magnum which is suspected to be the point of rupture (B).
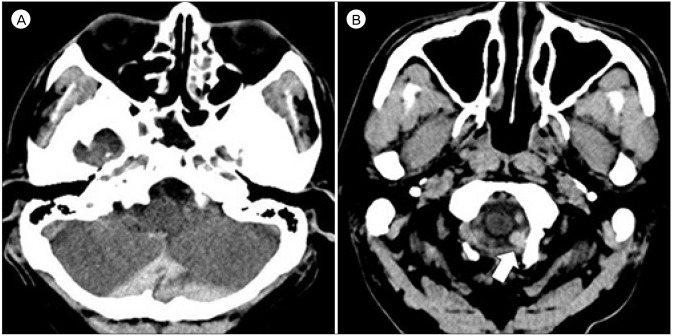
Fig. 2
Three-dimensional reconstruction (A), arterial phase (B), and venous phase (C) of left external carotid angiogram show a dural arteriovenous fistula at the foramen magnum fed by the hypoglossal branch (white arrowheads) of the neuromeningeal trunk (black arrows) of left ascending pharyngeal artery and drained into the suboccipital venous plexus and sigmoid sinus (long white arrows). The pharyngeal trunk (white arrows) and the odontoid arcade (black arrowheads) are also identified. Note the venous varix adjacent to the fistula.
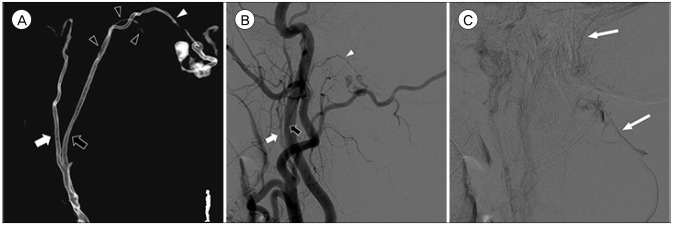
Fig. 3
Three-dimensional reconstruction (A), arterial phase (B) of the left external carotid angiogram at a working angle demonstrate a venous varix distal to the fistula. The hypoglossal branch (white arrowheads) of the neuromeningeal trunk (black arrows) is the only route that leads to the fistulous point. The pharyngeal trunk (white arrows) is overlapped on it. The odontoid arcade (black arrowheads) is also visible.
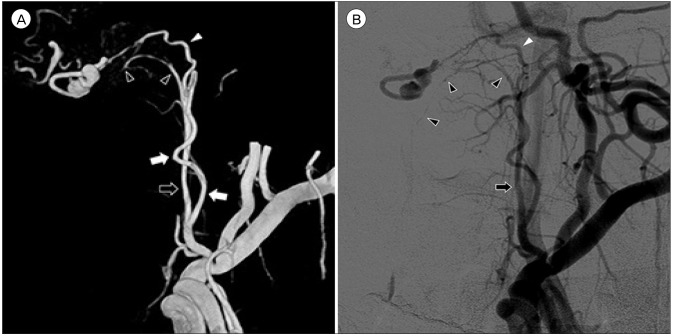
Fig. 4
A superselectvie angiogram clearly shows the configuration of the target (A). Note that the microcatheter should be located as close to the fistulous point as possible. After injecting 0.4 mL of Onyx (B), the fistulous point and the ruptured venous varix are occluded (C).
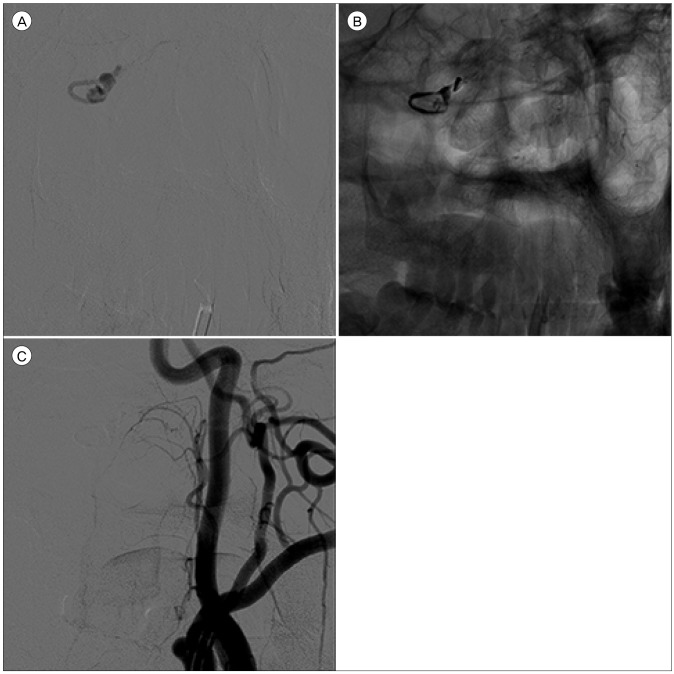
Table 1
Summary of reports in the literature on D-AVF at the foramen magnum fed by the APA
| Study | Sex/age | Presentation | Feeder | Direction of drainage | Treatment |
|---|---|---|---|---|---|
| Gaensler et al5) (1990) | M/50 | Myelopathy | VA, APA | Inferior | Embolization (PVA, IBCA) |
| Willinsky et al22) (1990) | M/57 | Myelopathy | APA | Both | Embolization (NBCA) |
| M/50 | Tinnitus, CN 6 palsy | APA, OA | Superior | Embolization (PVA) | |
| Mascalchi et al13) (1996) | M/69 | Myelopathy | APA, VA | Inferior | Surgery |
| M/53 | Myelopathy | APA | Inferior | Embolization (glue) | |
| Hurst et al9) (1999) | M/50 | Myelopathy | APA | Inferior | Embolization (PVA) |
| Lee et al11) (2010) | M/59 | Myelopathy | APA | Inferior | Embolization (NBCA) |
| Guo et al7) (2010) | M/35 | SAH | OA, APA | Both | Surgery |
| Spiotta et al17) (2011) | M/49 | Myelopathy | APA | Both | Embolization (Onyx) |
| Liang et al12) (2013) | M/55 | Myelopathy | APA, VA | Inferior | Embolization (Onyx) |
| F/49 | Myelopathy | OA, VA, APA | Inferior | Embolization (Onyx) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download