Abstract
Objective
Angioplasty and Stenting of intracranial atherosclerotic lesions have a higher complication rate and a large proportion of this is attributable to side branch arterial occlusion from forceful displacement of the atheroma into the ostia or snowplowing effect. This can result in severe disabilities when it result in small infarcts involving eloquent areas in the posterior circulation or the motor tracts.
Materials and Methods
We present a series of 6 cases utilizing a new dual catheter technique for maintaining the patency of at-risk vessels during angioplasty and stenting. There are several methods previously described to help reduce the incidence of stroke but because they do not have a physical presence in the ostia to protect it, they are unable to guarantee the patency of the vessel.
Results
All 6 patients underwent angioplasty and stenting with the technique. The patients were assessed for complications with post-procedure magnetic resonance imaging and no complications were found.
Conclusion
In this preliminary series, the dual catheter technique appears to safe and effective in preventing occlusion of the adjacent branch arteries. This technique may facilitate the use of the Wingspan stent in the treatment of intracranial atherosclerotic stenotic segments by reducing the risk of peri-procedural stroke.
Stroke is the leading cause of worldwide disability and intracranial atherosclerotic stenosis (ICAS) is an important cause of ischemic stroke among Asians, especially among Chinese populations.16) Where the proportion of ischemic stroke caused by ICAS can be up to 50%.15)16)17) Medical treatment is recommended for the treatment of symptomatic ICAS but despite our best efforts, the rate of pharmacological failure resulting in recurrent ischemic stroke can be 15-20%.2)9)12)
A major concern of intracranial angioplasty with or without stent placement has been occlusion of the perforator vessels such as the lenticulostriate arteries during middle cerebral artery angioplasty or posterior inferior cerebellar artery during posterior circulation stenting. The SAMPPRIS trial determined that stenting and angioplasty arm for ICAS has a higher rate of strokes than medical treatment arm. Moreover, the majority of the peri-procedural ischemic strokes in the trial were in perforator territories adjacent to the vessel the stent was deployed in.1) These strokes were presumably from the ‘snow-plowing’ effect where the atheromatous plaque is pushed into the small perforator vessels by balloon inflation from angioplasty or stent deployment. We present a novel dual catheter technique to preserve the patency of these adjacent perforator arteries that are at risk of being occluded during angioplasty and stenting of ICAS.
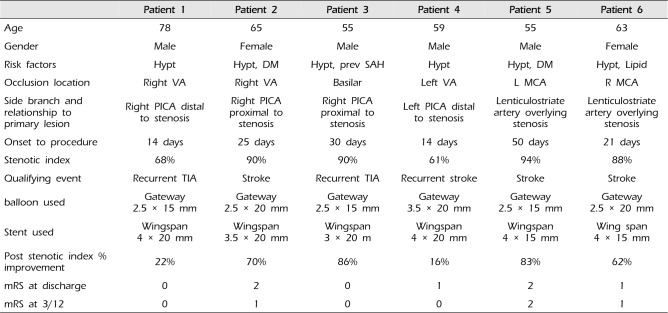
6 patients in 2015 with ischemic stroke despite best medical treatment (dual antiplatelet treatment with maximal doses of Atorvastatin and blood pressure medication) underwent angioplasty and stenting of the ICAS. Written informed consent was obtained from all 6 patients. The clinical characteristics of the 6 patients are summarized in Table 1. Of note 2 patients had moderate stenosis with a stenosis index of 61% and 68% however they underwent angioplasty and stenting because of their recurrent symptoms despite maximal medical therapy. All patients were treated preoperatively with a dual antiplatelet therapy of 150 mg of aspirin and 75 mg of clopidogrel daily for 5 days before the procedure. The effectiveness of the platelet inhibition therapy was tested by platelet activity assays, and in cases of insufficiency loading doses were administrated. The patients all underwent general anesthesia for the procedure and the clotting time was kept at 250-300 seconds by heparin.
Cerebral angiography was performed via a femoral approach. After arterial puncture a 7 F guiding sheath (Shuttle) and 7 F guiding catheter Neuron 088 (Penumbra, Alameda, CA, USA) was advanced to the base of the stenosis (Fig. 1A, B, 2A). A microcatheter (Echelon-10, Covidien) over a 0.0014 inch microguide wire (Transcend, Stryker Corporation, Kalamazoo, MI, USA) was forwarded through the 7 F guiding catheter and guided up and into the ostia of the adjacent perforator artery at risk. An important point that bears highlighting is that the microcatheter used should be the Echelon-10 (Covidien), otherwise, the Gateway balloon will have difficulty passing through the guide catheter when adjacent to the other microcatheter due to lack of space in the lumen. The microcatheter was then guided deeper into the perforator and a secure distal position achieved (Fig. 1C, 2B). The guidewire was removed and a selective angiogram was performed to ensure the microcatheter was in the correct vessel and that the purchase in the perforator was stable (Fig. 1C, D, 2C). This was left there to provide a physical presence in the ostia of the aforementioned perforator and keep it open.
For the posterior circulation, we tended to utilize a bilateral femoral puncture technique for a bilateral vertebral artery approach so that the catheters had no issues with accommodation or becoming wedged on top of each other. If the internal cerebral artery or vertebral artery was stenosed such that bilateral vertebral artery access was not practical, a 6 F guiding sheath (Shuttle) and a 6 F guide catheter (Envoy DA I.D. 0.071") or (Navien I.D. 0.072") was used instead.
The subsequent portion is similar to the standard Wingspan stent deployment with a few important differences. A second Echelon-10 microcatheter and Transcend guidewire was introduced into the same Neuron Max and the wire was advanced into and over the stenotic segment following. At this point the guidewire is removed and gateway balloon was advanced into position within the microcatheter and inflated. The atheroma dislocated likely piling up around the microcatheter in the side-branch which is keeping the ostia patent. To minimize the release of debris and emboli, the Wingspan stent was deployed first distal to the occlusion (thus trapping any debris that might be later released between the stent and the vessel wall), then through the stenotic segment, and finally just proximal to the occlusion, thus traversing the entire lesion. After the stent is deployed an angiographic run is then done to ensure that the side-branch is patent before the final microcatheter is removed (Fig. 1E, 2D, E).
Delayed angiographic runs were also performed to rule out thrombosis, spasm or dissection. Patients received a maintenance dose of aspirin (100 mg daily) and clopidogrel (75 mg daily) the day after the procedure.
All 6 patients underwent magnetic resonance imaging examination post procedure which showed no infarcts, or other procedure related complication such as vessel rupture or haemorrhage. They were assessed by an independent physician post procedure, daily during the admission and at 3 months after discharge with no new neurological deficits. The modified Rankin scale (mRS) at three months was 0-1 for all patients. A follow-up angiographic study at six months for was completed in all 6 patients which confirmed patency of the side branch, there was no restenosis in the stented vessels as well (Table 1).
Angioplasty and stenting is an important alternative modality for the treatment of intracranial artery stenosis but it remains challenging to ensure that the benefit outweighs the risks. We showcase a novel technique to occupy the ostia of the at-risk side-branch arteries during angioplasty and stenting of the ICAS, thereby preventing peri-procedural infarcts.
One major concern of intracranial stenting is side-branch occlusion from the “snow plowing” effect (i.e., forceful displacement of an atheroma in the side-branch ostia when stents or balloons expand through a stenosis) attributed to high deployment pressure of the balloon expansion stent.5)6) The stent struts crossing the ostia of side-branchs in the atherosclerotic vessel may be associated with occlusion of side-branchs after intracranial stenting.8) These vessels can remain occluded post-procedure, indefinitely extending the time of ischemia.
In the early history of ICAS, stenting was performed with the balloon expansion stents used for coronary vessels and it was previously reported that the diameter of the side branch was the most important factor contributing to side-branch occlusion when coronary stents were used. However, the occlusion of perforating arteries in the brain may result in significant neurologic deficits, despite the side branch arteries typically being much smaller than that of the coronary artery perforators.7)11) A large single center series of 181 patients with ICAS treated with balloon expandable coronary stents, patients with preprocedural infarction of territories fed by perforators had post-procedural infarction at a rate significantly higher than patients without pretreatment perforator-region infarctions.4) These earlier studies with coronary stents in ICAS showed the degree of risks that perforator rich regions face from snowplowing during angioplasty and stenting.
Several techniques were introduced to limit complications for the procedure, such as submaximal balloon dilatation or a staged approach to allow intimal fibrosis and vascular remodeling in the interim period, which may reduce the risk of acute perforator occlusion.5) Advances in stent technology produced the Wingspan device which performed better than the balloon-mounted stents in terms of navigability to the target site. The self-expanding stents were designed to cause less vasospasm and side-branch occlusions with submaximal dilatation,3) Nonetheless, the previously staged procedures are now combined and without an intervening time period to allow for intimal fibrosis and remodeling, the self-expanding stent can still dislodge the plaque and occlude the ostia. This was displayed in the SAMPPRIS trial with its higher incidence of stroke.
There are some technical precautions during the procedure that help to ensure that the residual stenosis is reduced as low as possible. Angioplasty is typically performed with a slow-graded inflation of the balloon to a pressure higher than nominal pressure. The self-expanding Wingspan stent exerts a continuous outward radial force against the vessel wall. This outward radial force prevents early vessel recoil and a stent with slightly larger diameter is usually chosen. Pathophysiologically when compared with coronary vessels, cerebral arteries lack a well-formed external elastic lamina and are relatively fixed from movement due to small branching arteries.13) All these factors contribute to the degree which atheroma is pushed into the ostia of adjacent vasculature.
We chose to use the 7 Fr Neuron to give the best balance of distal access and large enough lumen that can accommodate all the required equipment. The size of the microcatheters used with respect to the lumen of the guide catheter is important, we chose 2 echelon-10 microcatheters to ensure that they would be fine enough to fit within the 7 F neuron guide catheter and still have enough support and tractability. For the posterior circulation, we use a bilateral femoral puncture for a bilateral vertebral artery approach.
In a study examining risk factors for stroke with ICAS stenting, patients had a higher risk of stroke if they had a posterior circulation stenosis and if the initial qualifying event was a stroke or transient ischemic attack. This may imply that the mechanism of further stroke is related to the pre-existing atheroma by the snow-plow effect. This is especially relevant in the posterior circulation with its many important eloquent side-branches and perforators.10) In fact, side-branch and perforator infarction was the most common single complication related to stenting in the SAMMPRIS trial, accounting for half of all stenting complications and 15/21 ischemic incidents.1) Unfortunately there were insufficient details in the publications to determine if these complications were related to lenticulostriate or basilar branches that would be amenable to accommodating a microcatheter.
Using a second microcatheter to preserve a branch has been described during coil embolization of a posterior communicating artery aneurysm when there is significant aneurysmal involvement of a relatively small PCOM artery. This technique has not been described during angioplasty and stenting. The double catheter technique is especially suitable for preventing side-branch infarcts in the posterior circulation with vasculature that can accommodate a microcatheter. Perforators on the other hand are usually much smaller vessels such that catheterization is not feasible and this technique cannot be applied. Nonetheless, in our series, 4 out of 6 of our patients had stenosis in the posterior circulation with the PICA vessels at risk of occlusion which was avoided by our technique.
Even in the post-SAMPRIS era where angioplasty alone (without stenting) is being proposed as a plausible alternative treatment for ICAS which have failed medical therapy, our double catheter technique is still viable to protect side-branch arteries.14) We appreciate that this is a small series comprising only 6 patients and is neither conclusive of the safety or efficacy of the technique. Nonetheless it is a new method that can potentially reduce avoidable strokes.
References
1. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 9. 365(11):993–1003. PMID: 21899409.
2. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005; 3. 352(13):1305–1316. PMID: 15800226.

3. Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology. 2005; 3. 47(3):222–228. PMID: 15912418.

4. Jiang WJ, Srivastava T, Gao F, Du B, Dong KH, Xu XT. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology. 2006; 6. 66(12):1868–1872. PMID: 16801652.

5. Leung TW, Yu SC, Lam WW, Chan AY, Lau AY, Wong LK. Would self-expanding stent occlude middle cerebral artery perforators? Stroke. 2009; 5. 40(5):1910–1912. PMID: 19182082.

6. Levy EI, Chaturvedi S. Perforator stroke following intracranial stenting: a sacrifice for the greater good. Neurology. 2006; 6. 66(12):1803–1804. PMID: 16801640.

7. Lopes DK, Ringer AJ, Boulos AS, Qureshi AI, Lieber BB, Guterman LR, et al. Fate of branch arteries after intracranial stenting. Neurosurgery. 2003; 6. 52(6):1275–1278. discussion 1278-9. PMID: 12762872.

8. Masuo O, Terada T, Walker G, Tsuura M, Nakai K, Itakura T. Patency of perforating arteries after stent placement? A study using an in vivo experimental atherosclerosis-induced model. Am J Neuroradiol. 2005; 3. 26(3):543–548. PMID: 15760863.
9. Nahab F, Cotsonis G, Lynn M, Feldmann E, Chaturvedi S, Hemphill JC, et al. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008; 3. 39(3):1039–1041. PMID: 18239161.

10. Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009; 6. 72(23):2014–2019. PMID: 19299309.

11. Poerner TC, Kralev S, Voelker W, Sueselbeck T, Latsch A, Pfleger S, et al. Natural history of small and medium-sized side branches after coronary stent implantation. Am Heart J. 2002; 4. 143(4):627–635. PMID: 11923799.

12. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995; 1. 26(1):14–20. PMID: 7839388.
13. Schumacher HC, Khaw AV, Myers PM, Gupta R, Higashida RT. Intracranial angioplasty and stent placement for cerebral atherosclerosis. J Vasc Interv Radiol. 2004; 1. 15(1 Pt 2):S123–S132. PMID: 15101521.

14. Umont TM, Sonig A, Mokin M, Eller JL, Sorkin GC, Snyder KV, et al. Submaximal angioplasty for symptomatic intracranial atherosclerosis: a prospective Phase I study. J Neurosurg. 2016; 10. 125(4):964–971. PMID: 26745485.
15. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003; Mar-Apr. 22(2):106–117. PMID: 12656117.
16. Wong KS, Li H, Lam WW, Chan YL, Kay R. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke. 2002; 2. 33(2):532–536. PMID: 11823665.

17. Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke. 2006; 8. 1(3):158–159. PMID: 18706036.

Fig. 1
(A) Right Vertebral stenosis with at-risk PICA artery. (B) 3-D reconstruction of the stenosis and PICA. (C, D) Echelon-10 microcatheter advanced into PICA and left to protect it, superselective contrast injection by hand to confirm placement. (E) Final outcome after angioplasty and stenting, PICA is preserved. PICA = posterior inferior cerebellar artery.
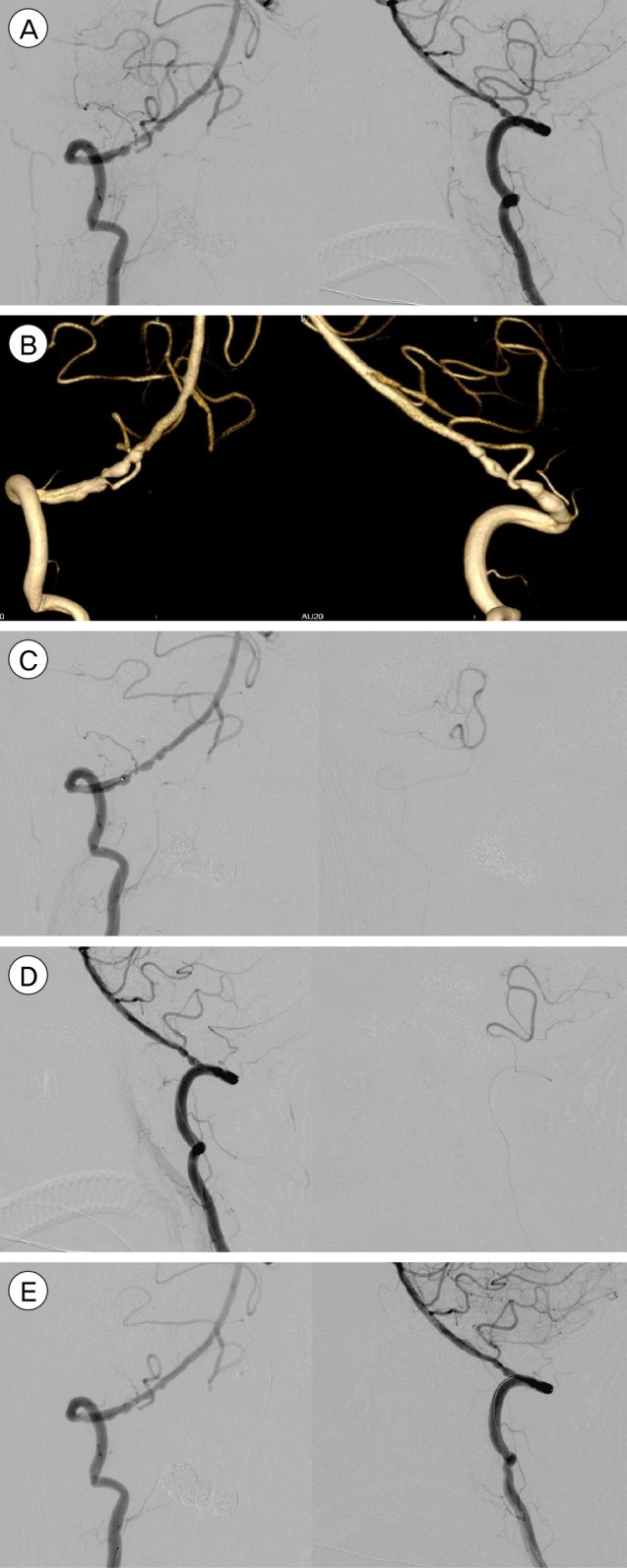
Fig. 2
(A) Right Vertebral stenosis with at-risk PICA artery. (B) Double microcatheters in R VA & PICA. (C) Double microcatheters in R VA & PICA with check angiographic run. (D) 3-D reconstruction post-angioplasty & stenting. (E) Final outcome after angioplasty and stenting, PICA is preserved. VA = vertebral artery; PICA = posterior inferior cerebellar artery.
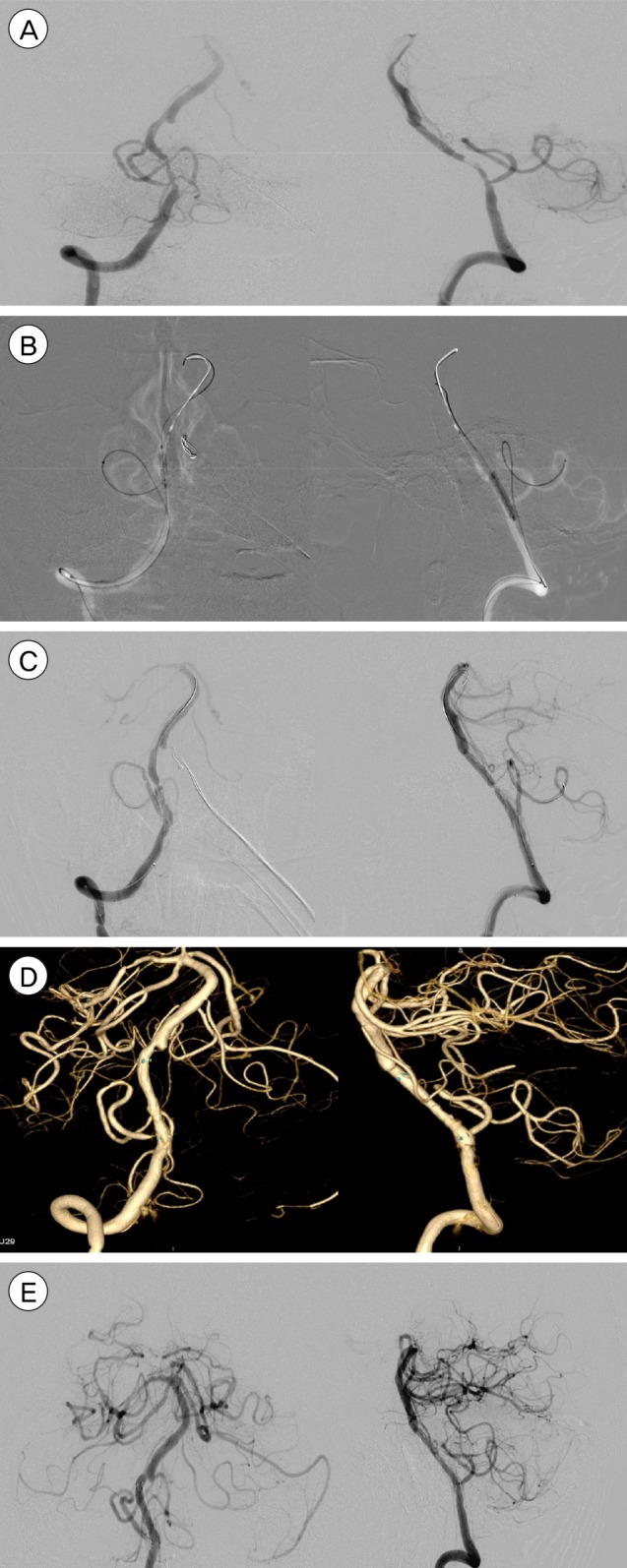
Table 1
Characteristics of patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download