Abstract
Objective
The authors evaluated the feasibility and targeting accuracy of CT fluoroscopy (CTF)-guided catheter placement and aspiration of intracerebral hematoma (ICH)s.
Materials and Methods
Nine patients (mean age, 63.3 ± 15.3 years) were treated by CTF-guided hematoma aspiration under local anesthesia. The targeting errors in the lesion center, volume of the aspirated hematoma, accuracy of the final catheter position, procedure time, and clinical outcomes were evaluated.
Results
All catheters were successfully placed in the center of the hematoma. The mean volume of the aspirated hematoma was 20.6 ± 8.8 mL (pre-treatment, 44.7 ± 20.1 mL; post-treatment, 24.1 ± 13.8 mL). The average procedure time was 25.1 minutes (range, 18-32 minutes). In one case with a scanty residual hematoma, the catheter was removed at the end of the procedure. In the remaining eight cases, the catheter was left in the residual hematoma for drainage and all catheter tips were accurately located in the final position. There were no procedure-related complications, including rebleeding and infection.
Catheter placement for intracerebral hematoma (ICH) aspiration with subsequent lysis is usually performed under the guidance of frame-based or frameless stereotaxy. For these stereotactic methods, several preparatory steps are required, including rigid frame or fiducial marker attachment, followed by image acquisition, image registration, and calculation of the target point. These processes are often time-consuming and inconvenient. In uncooperative, irritable patients, deep sedation or general anesthesia may be necessary. Above all, it is basically a blind intervention, i.e., the operator must rely on the images obtained before ICH aspiration, and how much and which part of the hematoma remains cannot be confirmed during the intervention.
For years, we have performed catheter-based ICH aspiration under CT fluoroscopy (CTF) guidance. The preparatory processes were simple, and rigid fixation was not necessary. More importantly, real-time information on the brain and ICH was available during the procedure. We report herein on the technique of CTF-guided ICH aspiration and its outcomes.
This study was approved by our institutional review board, and the requirement to obtain written informed consent to participate in this study was waived. Patients with a spontaneous ICH > 30 mL in volume who had significant neurologic symptoms secondary to the ICH were considered eligible for CTF-guided ICH aspiration. To exclude other causes of hemorrhage, including cerebral aneurysms, arteriovenous malformations, or any other vascular anomalies, CT angiography, MR angiography, or digital subtraction angiography was obtained on all patients. Patients with signs of herniation were excluded and treated by open surgery. In some cases, CTF-guided ICH aspiration was performed on patients whose relatives refused craniotomy. Since 2008, CTF-guided ICH aspiration was performed in 9 patients.
We used a 64-channel CT scanner (Brilliance; Philips Medical Systems, Best, the Netherlands) for the procedure. The patient was placed supine on the CT table with monitoring of vital signs (IntelliVue MP 60; Philips Medical Systems, Best, the Netherlands). The catheter entry point was selected according to the location of the ICH, as follows: supraorbital point (4-5 cm above the orbital rim in the plane of the pupil)3) for putaminal hemorrhage (Fig. 1), Keen's point (2.5-3 cm posterior and 2.5-3 cm superior to the pinna)3) for thalamic hemorrhage, the most superficial point in the hematoma for lobar hemorrhage (Fig. 2), and suboccipital area above the hematoma for cerebellar hemorrhage (Fig. 3). After placing a radio-opaque marker (needle or fiducial marker) at the entry point, initial CT scan images were acquired. Based on these CT scan images, the CT gantry was angled at a line between the radio-opaque marker and the lesion center, from which the entire process of the catheter insertion could be observed in real time on a LCD monitor in the CT room as the catheter path coincided with the imaging plane. The hair was shaved around the entry point (5 × 5 cm) and a drape was placed using the usual aseptic technique. After administration of local anesthesia, a 1 cm skin incision was made at the entry point and a small burr hole was made using a twist hand drill. Under CTF guidance, a 12-Fr silicon catheter (Youshin Medical, Seoul, Korea), which is used for external ventricular drainage was inserted into the center of the hematoma, followed by careful manual aspiration of the hematoma using a 10 mL syringe. To facilitate hematoma removal, the location of the catheter tip was adjusted within the hematoma. The process of catheter insertion, adjustment of the catheter tip, and removal of the hematoma could be observed in real time with a CT room monitor. The exposure parameters were 120 kV and 60 mAs, with a slice thickness of 3 mm. One-to-four CT image cuts (not a full package of brain CT images) per fluoroscopy were obtained; three-to-four brief fluoroscopic shots (one shot for initial catheter insertion into hematoma and additional shots to assess the residual hematoma or adjust the catheter tip) were necessary to complete the entire process. We completed the procedure when the CT showed relief of the mass effect of the hematoma, even though a small amount of hematoma remained. Before finishing, whole brain CT scan was performed for detection of any procedural complications at other sites.
In cases with a residual hematoma, the catheter was left for drainage and connected to a closed external drainage system. The final location of the catheter tip in the hematoma cavity was also confirmed under the guidance of CTF. The residual hematoma was drained naturally. However, when the catheter lumen was blocked by a clot and the hematoma could not be drained despite gentle squeezing of the catheter, a fibrinolytic agent was injected. Urokinase (4,000 IU; Greencross Health Care, Yongin, Korea) was administered through the catheter, which was subsequently flushed with 2 mL of saline, and then clamped. After 30 minutes the catheter was unsealed and the liquefied hematoma was drained. The catheter was removed when a follow-up CT scan showed < 10 mL of the ICH volume or the patient showed marked improvement. Prophylactic antibiotics (cefazolin 2 g bid) were maintained during drainage of the hematoma in cases where the catheter was left.
We retrospectively reviewed the medical records of the 9 patients, including the clinical records (Glasgow coma scale score at the time of admission and modified Rankin scale score 6 months after surgery) and radiologic data (side, location, and volume of the ICH). The radiologic images were reviewed by a neuroradiologist (S.H. Kim). The ICH volume was measured on a PACS workstation using free-hand region of interest measurement tools (Infinnit, Seoul, Korea). After all lesion areas were segmented manually on a slice-by-slice basis, the total volume was calculated as the product of the slice thickness and the total lesion area.
To evaluate outcomes, we reviewed the procedure time (from scout CT scan to final CT scan), the number of targeting errors in the lesion center at the catheter insertion, the volume of aspirated hematoma (the difference between the pre-treatment ICH volume and post-treatment CT), and the final catheter position, which was considered accurate when the catheter tip was placed in the center of the parenchymal clot two-thirds the length of the long axis and within the middle one-third of the clot.5) Procedure-related complications, including rebleeding and infection, were also evaluated.
The patients treated by CTF-guided ICH aspiration included 6 men and 3 women. The mean age was 63.3 years (range, 33-81 years; Table 1). Oropharyngeal intubation was performed before the procedure in 4 patients (cases 1, 2, 4, and 6) and ambu bagging was required in 2 patients (cases 1 and 4) during the procedure. However, manual ambu bagging did not disturb the procedure. Midazolam and a short-acting muscle relaxant were used for the patients requiring intubation. In the remaining cooperative 5 patients (cases 3, 5, 7, 8, and 9), the procedure was performed without sedation.
The initial ICH volumes ranged from 28.4-93.1mL with a mean of 44.7 mL (Table 1). In all cases, the catheter was easily introduced into the center of the hematoma with a single pass using one CT fluoroscopy shot. The mean volume of the aspirated ICH was 20.6 mL (range, 8.6-35.9 mL) and the residual ICH volume measured an average of 24.1 mL (range, 6.2-57.2 mL) after aspiration of the ICH. In case 1, with scanty residual hematoma, the catheter was removed at the end of the procedure. In the remaining 8 cases, the catheter was left in the residual hematoma and the catheter tip was placed in the final accurate location, which we intended to perform under real-time observation with CTF. The procedure took an average of 25.1 minutes (range, 18-32 minutes).
Post-operatively, of the 8 cases in which the catheter was left in the hematoma cavity, the residual hematoma was drained without clot lysis with urokinase in 3 cases (cases 3, 7, and 8). In the remaining 5 cases, clot lysis was required because of blockage of the catheter lumen by a clot during drainage of the residual hematoma. Clot lysis was performed ≤ 2 times (≤ 8,000 IU of urokinase), except in 1 patient (case 6, 20,000 IU of urokinase, 5 times) with a large amount of residual hematoma.
There were no procedure-related complications. There were 2 mortalities (case 1 secondary to pneumonia; and case 2 secondary to an acute MI), but no procedure-related deaths. The remaining 7 patients were discharged from the hospital. The patients' 1-year follow-up clinical outcomes are summarized in Table 1.
CTF has been widely used for percutaneous interventions, such as biopsy and drainage procedures. However, CTF-guided ICH aspiration has received little attention as a minimally invasive technique, despite the success of an early study.2) Unlike frame-based or other frameless stereotactic ICH aspiration, it does not require complicated preparations and rigid fixation. General anesthesia was not necessary even in uncooperative, irritable patients. We were able to confirm the catheter position on a real-time basis. Accurate catheter position facilitates effective aspiration and drainage of the hematoma, and lysis of the clot with a small amount of fibrinolytics.7)8) In addition, using CTF we were able to see whether or not the mass effect was relieved during intervention. The surgeon can use this information in real-time and make a decision on the end-point of the procedure.
When the initial catheter location was not adequate for aspiration of a sufficient amount of hematoma, we were able to reposition the catheter tip to the exact part of the remaining hematoma. We did not need to take risks in performing it blindly. In addition, when the procedure was completed, the final catheter position could be assured. We could avoid targeting error and improper catheter position, which may lead to treatment failure, and even damage other brain structures. CTF-guided ICH aspiration also shortens the procedure time. In our experience, it was performed within an average of 30 minutes.
Catheter placement and aspiration of ICH using conventional CT guidance was previously reported.4) In that study Kocher's point (3 cm from midline and 1cm anterior to coronal suture) was used as an entry point for deep ICH, which is usually used in other stereotactic catheter insertion methods for deep ICH. However, for the catheter path to the lesion center to coincide with the image plane, we used the supraorbital point for putaminal hemorrhage or Keen's point for thalamic ICH instead of Kocher's point. When using a standard Kocher's point, it is difficult to put the CT gantry angle at a line between Kocher's point and the hematoma center because that line is outside of CT gantry working range. Accordingly, in their report, repeated conventional axial CT scans were necessary to assess the catheter path to the hematoma during catheter placement. However, the supraorbital and Keen's point can provide a comfortable work space without the disturbance caused by the CT gantry during the procedure and fit the catheter path for the imaging plane to visualize the entire process of catheter insertion in real time. These points are also the safe entry points which have long been used for external ventricular drainage in neurosurgery.3)
The major limitation of CTF-guided ICH aspiration is radiation exposure of patients and surgeons. It can be reduced by brief use of CTF and low fluoroscopic parameters.1)6) Because hematoma removal is observed in real time, the surgeon may attempt complete removal of the hematoma, which could lead to rebleeding. Therefore, ICH aspiration must be limited to relief of the mass effect.
A catheter navigation and ICH aspiration with CTF is a feasible procedure shortening the time required for the procedure. Through the observation in real time on the monitor, CTF enables accurate catheter position, which facilitates effective aspiration and drainage of the ICH with a small amount of fibrinolytics. Also, the operator can determine the end-point of the procedure with the real time CT information. Our results suggest that CTF could substitute for stereotactic methods.
ACKNOWLEDGEMENT
This study was supported by grants from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020), the Seoul National University Bundang Hospital research fund (02-2013-122), and a contribution from Jinyoung TBX Co., LTD (06-2013-080).
References
1. Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001; 5. 219(2):515–520. PMID: 11323481.

2. Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996; 9. 200(3):851–856. PMID: 8756943.

3. Mapstone TB, Ratcheson RA. Techniques of ventricular puncture. In : Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGrow-Hill;1996. p. 179–180.
4. Montes JM, Wong JH, Fayad PB, Awad IA. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma : protocol and preliminary experience. Stroke. 2000; 4. 31(4):834–840. PMID: 10753984.
5. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008; 105:147–151. PMID: 19066101.

6. Murphy K, Nussbaum DA, Gailloud P. CT fluoroscopy: novel application for the treatment of ventricular pathologies. Neuroradiology. 2007; 4. 49(4):373–378. PMID: 17393194.

7. Niizuma H, Otsuki T, Johkura H, Nakazato N, Suzuki J. CT-guided stereotactic aspiration of intracerebral hematoma--result of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985; 48(1-6):427–430. PMID: 3915660.
8. Schaller C, Rohde V, Meyer B, Hassler W. Stereotactic puncture and lysis of spontaneous intracerebral hemorrhage using recombinant tissue-plasminogen activator. Neurosurgery. 1995; 2. 36(2):328–333. discussion 33-5. PMID: 7731513.

Fig. 1
Case 5. A large amount of hematoma is observed in the right putamen on pre-treatment computed tomography (CT) (55.5 mL, A). Using CT fluoroscopy, a 12-Fr silicon catheter can be placed in the center of hematoma (B). After the procedure, post-treatment CT scan (C) shows the small amount of residual hematoma (23.5 mL), which was successfully drained with a single urokinase (4,000 IU) injection via the catheter.
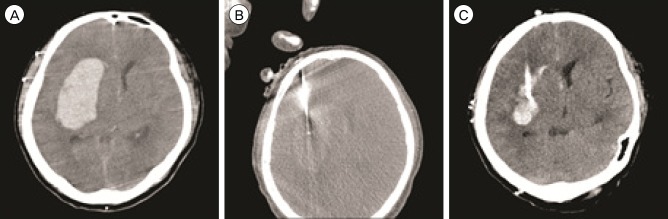
Fig. 2
Case 3. Using computed tomography (CT) fluoroscopy, a 12-Fr silicon catheter is advanced into the hematoma in the right frontal lobe (34.1 mL, A and B). Post-treatment CT scan (C) shows relief of the mass effect of the hematoma. Urokinase was not necessary for the residual hematoma (17.4 mL).
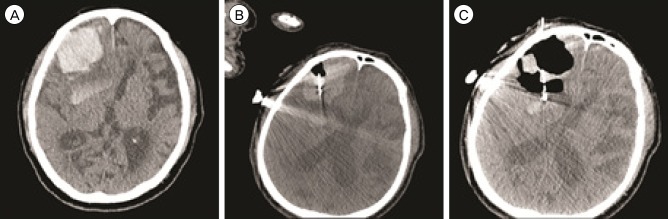
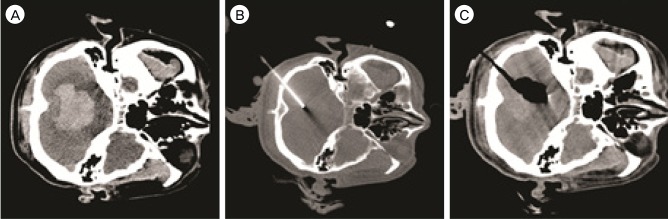
Fig. 3
Case 1. The initial computed tomography (CT) scan shows a right cerebellar hematoma extending to the 4th ventricle measuring 28.4 mL in volume (A). Under CT fluoroscopy guidance, a 12-Fr silicon tube is inserted through a small burr hole in the suboccipital area (B). After aspiration of the hematoma, a small amount of hematoma (6.2 mL) which did not require subsequent lysis with fibrinolytics is shown on post-treatment CT scan (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download