Abstract
Spontaneous anterior cerebral artery (ACA) dissection, although extremely rare, is often associated with severe morbidity and mortality. It could lead to cerebral hemorrhage, ischemic stroke, or, rarely, combination of hemorrhage and ischemia due to hemodynamic changes. Prompt and accurate diagnosis is essential for determining the appropriate management. However, the optimal treatment for ACA dissection remains controversial. Herein, we report on two rare cases of subarachnoid hemorrhage (SAH) caused by ACA dissection; a case presenting with simultaneous SAH and infarction without aneurysmal formation and another case presenting with SAH with fusiform aneurysmal formation. A review of the related literature is provided, and optimal treatments for each type of dissection are suggested.
Spontaneous intracranial arterial dissection usually occurs in the posterior circulation and is relatively rare in the anterior circulation, particularly the anterior cerebral artery (ACA).4)7)8)17)20) It tends to develop in young healthy people, and is often associated with severe morbidity and mortality.13)17) It can also cause cerebral hemorrhage, ischemic stroke, or, rarely, combination of hemorrhage and ischemia.13)17)
The exact incidence of ACA dissection has not been reported. Isolated ACA territory infarction is very rare, representing 0.5-3% of all cases of ischemic stroke, and 47% of cases are angiographically proven dissection confined to the ACA.8)10)14)15)18) The number of patients diagnosed with spontaneous ACA dissection has recently shown a marked increase due to advancement of diagnostic devices, such as multi-channel 3-dimensional computed tomography angiography (3D-CTA), high Tesler magnetic resonance angiography (MRA), and high quality digital subtraction angiography (DSA).7)13) In addition to the aid of advanced diagnostic tools, a high level of suspicion should be maintained in order to prevent misdiagnosis. Due to the high instability of the dissected arterial walls, any aggressive manipulations or those made without preparation by careless decision could have a devastating result by arterial rupture.
Herein, we report on two rare cases of subarachnoid hemorrhage (SAH) caused by ACA dissection; a case presenting with simultaneous SAH and infarction without aneurysmal formation and another case presenting with SAH with fusiform aneurysmal formation.
A 48-year-old female presented with a sudden bursting headache and right hemiparesis of grade I/V. Initial brain computed tomography (CT) showed an obvious SAH at the anterior interhemispheric fissure and left superior frontal sulcus (Fig. 1A). Conventional cerebral angiography showed abrupt luminal tapering of the left distal ACA with compromised distal flow (Fig. 1B, C). Her brain magnetic resonance imaging (MRI) showed an acute cerebral infarction in the left ACA vascular territory (Fig. 1D).
Conservative treatment was administered since the area of infarction matched the presumed territory of the occluded ACA without penumbra zone and further revascularization might not guarantee any benefit. Three months later, she was able to walk unassisted with minor motor weakness of grade IV/V on her right leg. She underwent repeated follow-up cerebral angiographies which showed remarkable arterial healing. Follow-up angiography after 33 months showed complete luminal healing without aneurysmal formation (Fig. 1E, F).
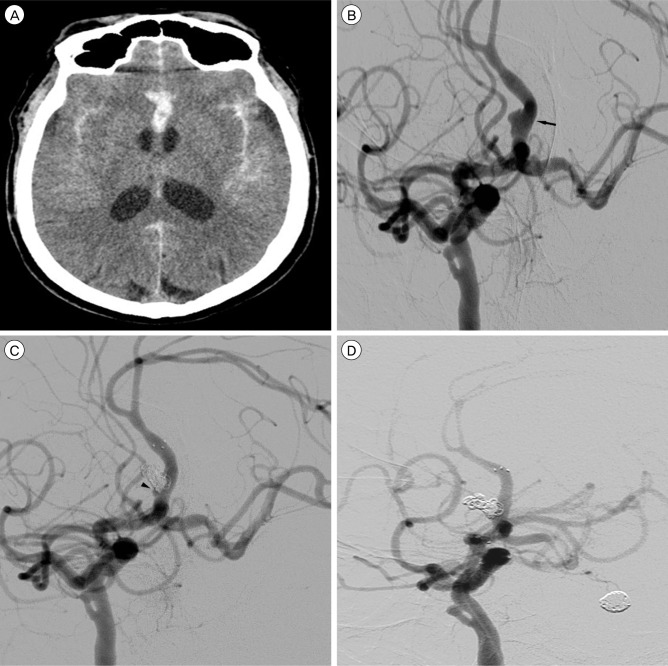
A 56-year-old male presented with deep stuporous consciousness with a Hunt and Hess grade of IV. Initial brain CT showed a thick SAH at the basal cistern, sylvian fissures, and anterior interhemispheric fissure (Fig. 2A). Conventional cerebral angiography showed an azygous ACA, chronic total occlusion of the left proximal internal carotid artery (ICA), and a fusiform aneurysm on the right side wall of the A2 segment (Fig. 2B). An arterial fenestration was also observed at the cavernous ICA. The aneurysm measured 6.25×2.86 mm in size on the 3-dimensional rotational angiogram with a neck size of 6.25 mm and height of 2.02 mm. The distal ACA flow remained patent without stenosis or occlusion.
Stent-assisted coil embolization of the azygous A2 dissecting aneurysm was performed under general anesthesia. A tiny remnant was left at the proximal portion (Fig. 2C), however, follow-up angiography after two weeks showed complete obliteration of the aneurysm with preservation of the parent artery (Fig. 2D). He showed improvement to drowsy mentality, but remained in a wheelchair-bound state.
Intracranial arterial dissection is rare, and approximately 90% of arterial dissections occur in the posterior circulation.7)12)18)19)20) Arterial dissections in the posterior circulation usually present with SAH because of their elongated subarachnoid course.1)6) In contrast, those of the anterior circulations are generally involved in the supraclinoid segment of the ICA and the middle cerebral artery and present with either hemorrhage or ischemia. ACA involvement is possible when the ICA dissection extends distally, however, those confined solely to the ACA are extremely rare.
The cause of arterial dissection is unknown. Several predisposing risk factors include preceding trauma and collagen disorders such as cystic necrosis of the media, fibromuscular dysplasia, moyamoya disease, Marfan's syndrome, Ehler's Danlos syndrome type IV, and so forth. Syphilitic angiopathy, Guillain-Barre syndrome, and common vascular risk factors such as atherosclerosis, hypertension, diabetes mellitus, and hyperlipidemia have also been implicated in the pathogenesis of arterial dissection.1)4)5)11)
Intracranial dissections can be categorized according to two types on the basis of the histological features; subintimal and subadventitial dissection. Subintimal dissection between the internal elastic lamina and media usually results in stenosis or occlusion with thrombosis of the true lumen of the parent artery, which causes ischemia.2)7)14)16)21) It generally shows good recovery from neurological symptoms. On the other hand, subadventitial dissection between the media and adventitia usually results in formation of aneurysmal dilatation and rupture of the dissected wall, which causes hemorrhage.1)7)14)18)21) The outcome is variable or worse than the ischemic presentation. In cases of simultaneous hemorrhage and ischemia, both mechanisms of subintimal and subadventitial dissection may be involved, indicating presentation of a more severe and deeper dissection plane, which may result in recurrent hemorrhage or ischemia.14) According to previous reports, ruptured ACA dissection often led to nonperimesencephalic SAH because distal ACA is located away from the circle of Willis at the basal cistern, and nonperimesencephalic SAH usually showed a good outcome.3)18) Therefore, we believe that SAH from ACA dissection often shows a relatively good outcome, if the hemorrhage is confined to the anterior interhemispheric fissure, when compared with SAH from aneurysms at the circle of Willis or dissecting aneurysms at the posterior circulation.
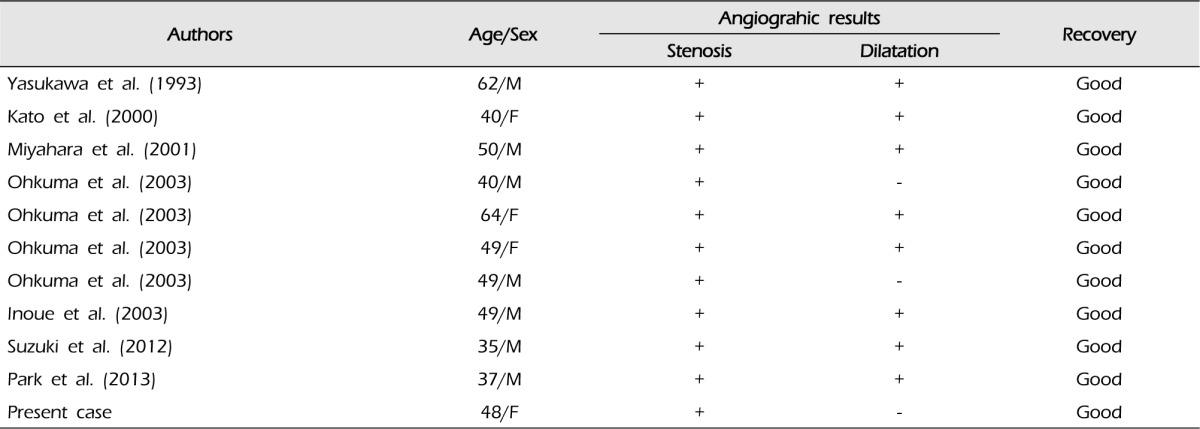
Reported incidence of arterial dissections with ischemic presentations is slightly higher than that of those with hemorrhagic presentations. Approximately 60% of patients with arterial dissections presented with clinical manifestations of cerebral ischemia and 30% with hemorrhagic events.17) Simultaneous hemorrhage and ischemia caused by ACA dissection, as in our first case, appears to be even more rare.9)20) To the best of our knowledge, 10 cases have been reported so far, and our case of simultaneous hemorrhage and ischemia caused by ACA dissection appears to be the eleventh report (Table 1).7)9)13)14)18)20) Eight of these 11 cases demonstrated stenoses with aneurysmal dilatations which account for occurrences of simultaneous hemorrhage and ischemia. The three remaining cases, including our case, demonstrated only stenoses, however, the possibility that the dilated segment may be too small to recognize or that it may not be visualized from rapid formation of intramural hematoma remains.
Diagnosis of ACA dissection requires a strong suspicion. Many clinicians have emphasized that it should be regarded as the differential diagnosis in case of acute stroke, especially when it occurs in younger adults, because ACA dissection frequently occurs in the middle-aged group and the mean age of these patients is 49.2 ± 10.4 years.13) Typical radiological evidence of dissection includes pearl and string sign (focal narrowing with distal dilatation), flame sign (tapered occlusion), occlusion, and pseudoaneurysm.1)11)13)14)15) Pathognomonic signs such as double lumen, or intimal flap are rarely observed.1)13)14) Besides, dynamic changes on serial angiography are the most important features of dissection.1)13)14) In our report, conventional angiography of both patients showed double lumen sign or dynamic changes on serial angiography.
The optimal treatment of ACA dissection remains controversial due to rare prevalence of dissection confined to the ACA, and is aimed at limiting neurological deficit by restoring blood flow and preventing occurrence of further ischemic or hemorrhagic events.7)13)14)18)19) Patients presenting with ischemia are indicated for conservative treatment if there are no salvageable penumbra areas. Another option is pushing the dissection flap outward in radial fashion using a stent if restoration of luminal patency is required.14) Patients with hemorrhage usually require surgical management including either direct surgical management or endovascular intervention in order to prevent rebleeding. In our study, the patient (Case 2) who suffered from SAH with evident aneurysmal formation underwent urgent endovascular intervention in order to seal off the leakage point and to prevent further recurrent hemorrhage. For patients presenting with simultaneous hemorrhage and ischemia, detailed evaluation of angiographic findings is required. Due to possible involvement of both subintimal and subadventitial dissections, false lumen and intimal flap may be larger than those in other types and higher rates of recurrent hemorrhage or ischemia are expected. Patients with arterial stenosis but without aneurysmal dilatation often receive conservative treatment and those with stenosis also with aneurysmal dilatation may require more aggressive treatments such as surgical or endovascular occlusion with or without bypass.7)14)18) However, variable degrees of spontaneous arterial repair may occur by collagen proliferation in all three types of dissections.7) In our report, the patient (Case 1) who suffered from SAH and infarction without aneurysmal formation initially received conservative treatment. Follow-up angiography showed considerable luminal healing without aneurysmal formation, and surgical procedures were not necessary. If follow-up angiography showed significant morphological changes of the ACA, surgical or endovascular management might have been required.
Spontaneous ACA dissection is extremely rare, and often develops in young adults. Detailed evaluation of 3D-CTA, MRA, and DSA is mandatory for prompt and accurate diagnosis. Consequences of an improper approach could be tragic, such as perforation of the arterial wall. The optimal treatment of ACA dissection remains controversial; however, the most reasonable option should be selected based on the clinical presentations and angiographic findings.
References
1. Amagasa M, Sato S, Otabe K. Posttraumatic dissecting aneurysm of the anterior cerebral artery: case report. Neurosurgery. 1988; 8. 23(2):221–225. PMID: 3141829.

2. Brandt T, Orberk E, Weber R, Werner I, Busse O, Muller BT, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001; 7. 57(1):24–30. PMID: 11445623.

3. Gupta SK, Gupta R, Khosla VK, Mohindra S, Chhabra R, Khandelwal N, et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg Neurol. 2009; 5. 71(5):566–571. PMID: 18617230.

4. Guridi J, Gallego J, Monzon F, Aguilera F. Intracerebral hemorrhage caused by transmural dissection of the anterior cerebral artery. Stroke. 1993; 9. 24(9):1400–1402. PMID: 8362439.

5. Hatayama K, karasawa H, Naito H, Hirota N, Sugiyama K, Ueno J, et al. Anterior cerebral artery dissecting aneurysm associated with fibromuscular dysplasia (FMD): a case report. No Shinkei Geka. 2001; 5. 29(5):451–456. Japanese. PMID: 11449718.
6. Huang YC, Chen YF, Wang YH, Tu YK, Jeng JS, Liu HM. Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol. 2009; 12. 72(Suppl 2):S20–S27. discussion S27. PMID: 19150115.

7. Inoue T, Fujimura M, Matsumoto Y, Kondo R, Inoue T, Shimizu H, et al. Simultaneous occurrence of subarachnoid hemorrhage and cerebral infarction caused by anterior cerebral artery dissection treated by endovascular trapping. Neurol Med Chir (Tokyo). 2010; 50(7):574–577. PMID: 20671384.
8. Kang SY, Kim JS. Anterior cerebral artery infarction: stroke mechanism and clinical-imaging study in 100 patients. Neurology. 2008; 6. 70(24 Pt 2):2386–2393. PMID: 18541871.

9. Kato N, Yamada Y, Hyodo A, Nose T. A case of anterior cerebral artery dissection presenting subarachnoid hemorrhage and cerebral infarction. Jpn J Neurosurg. 2000; 9(3):157–161. Japanese.

10. Kazui S, Sawada T, Naritomi H, Kuriyama Y, Yamaguchi T. Angiographic evaluation of brain infarction limited to the anterior cerebral artery territory. Stroke. 1993; 4. 24(4):549–553. PMID: 8465361.

11. Kim YW, Yoo SH, Kim SR, Kim SD, Park IS, Baik MW. Dissecting aneurysm at the A1 segment of the anterior cerebral artery manifesting as subarachnoid hemorrhage. Korean J Cerebrovasc Surg. 2005; 12. 7(4):324–328.
12. Lee MS, Lee YS, Lee JH, Ryu KY, Kang DG. A case of acute basilar artery dissection associated with sexual intercourse: a life-threatening cause of coital cephalgia. J Korean Soc Intravasc Neurosurg. 2011; 6(1):42–46.
13. Ohkuma H, Suzuki S, Kikkawa T, Shimamura N. Neuroradiologic and clinical features of arterial dissection of the anterior cerebral artery. AJNR Am J Neuroradiol. 2003; 4. 24(4):691–699. PMID: 12695205.
14. Park YK, Yi HJ, Lee YJ, Kim YS. Spontaneous anterior cerebral artery dissection presenting with simultaneous subarachnoid hemorrhage and cerebral infarction in a patient with multiple extracranial arterial dissections. J Korean Neurosurg Soc. 2013; 2. 53(2):115–117. PMID: 23560177.

15. Sato S, Toyoda K, Matsuoka H, Okatsu H, Kasuya J, Takada T, et al. Isolated anterior cerebral artery territory infarction: dissection as an etiological mechanism. Cerebrovasc Dis. 2010; 1. 29(2):170–177. PMID: 19955742.

16. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001; 3. 344(12):898–906. PMID: 11259724.

17. Suzuki I, Nishino A, Nishimura S, Numagami Y, Suzuki H, Utsunomiya A, et al. Nontraumatic arterial dissection of the anterior cerebral artery: six cases report. No To Shinkei. 2005; 6. 57(6):509–515. Japanese. PMID: 16026047.
18. Suzuki K, Mishina M, Okubo S, Abe A, Suda S, Ueda M, et al. Anterior cerebral artery dissection presenting subarachnoid hemorrhage and cerebral infarction. J Nippon Med Sch. 2012; 79(2):153–158. PMID: 22687360.

19. Yamaura A, Ono J, Hirai S. Clinical picture of intracranial non-traumatic dissecting aneurysm. Neuropathology. 2000; 3. 20(1):85–90. PMID: 10935444.

20. Yasukawa K, Kamijo Y, Momose G, Kobayashi S, Ikeda A. A case of anterior cerebral artery dissecting aneurysm presenting subarachnoid hemorrhage and cerebral infarction at the same time. Surg Cereb Stroke. 1993; 21:461–466. Japanese.

21. Yonas H, Agamanolis D, Takaoka Y, White RJ. Dissecting intracranial aneurysms. Surg Neurol. 1977; 12. 8(6):407–415. PMID: 594878.
Fig. 1
Initial brain computed tomography (CT) scan shows an obvious subarachnoid hemorrhage (SAH) at the anterior interhemispheric fissure and left superior frontal sulcus (A). Anteroposterior (AP) view (B) and lateral view (C) of the left internal carotid angiogram show abrupt luminal tapering (black arrows) of the left distal anterior cerebral artery (ACA) with compromised distal flow. Diffusion-weighted magnetic resonance imaging (MRI) shows an acute cerebral infarction in the left ACA vascular territory (D). AP view (E) and lateral view (F) of the left internal carotid angiogram after 33 months show complete luminal healing (black arrowheads) without any aneurysmal formations.
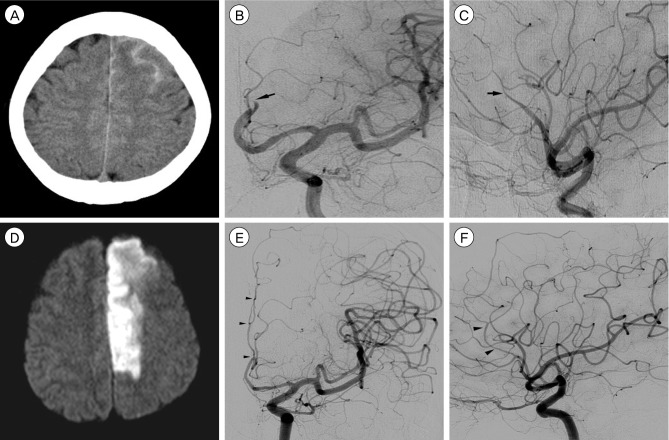
Fig. 2
Initial brain CT scan shows a SAH at the sylvian fissures and anterior interhemispheric fissure (A). Left anterior oblique view (B) of the right internal carotid angiogram shows a fusiform aneurysm (black arrow) on the right side wall of the A2 segment of azygous ACA. The distal ACA flow remains patent without stenosis or occlusion. Note a suspicious double lumen sign at the base of fusiform dilatation. Post-procedural angiogram (C) shows a tiny remnant (black arrowhead) at the proximal portion. Follow-up angiogram after two weeks shows complete obliteration of the aneurysm with preservation of the parent artery (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download