Abstract
Treatment options of ruptured vertebrobasilar fusiform aneurysms (VFA) are limited and often carry significant mortality and morbidity. We report the use of Pipeline Embolization Device (PED) to successfully treat a patient with a ruptured vertebrobasilar fusiform aneurysm (VFA) who presented with subarachnoid hemorrhage (SAH). A 73 year-old man with a history of cardiac stent placement seven days earlier presented with Hunt-Hess II SAH. He was taking aspirin and clopidogrel. Computed tomography angiogram revealed a large vertebrobasilar fusiform aneurysm. Microsurgical treatment options are technically challenging and carry high risk. He underwent endovascular treatment of the ruptured VFA using overlapping PEDs. Five PEDs were placed in a telescoping fashion to reconstruct the affected portions of the left vertebral and basilar arteries. An additional 2-mm blister aneurysm in the right vertebral artery was also discovered during the conventional cerebral angiography and was treated with one additional PED. The patient remained neurologically intact after the procedure. He was continued on aspirin and clopidogrel. Follow-up magnetic resonance imaging at three months demonstrated patency of the stents without any evidence of ischemic change. Follow-up conventional cerebral angiogram at six months demonstrated thrombosis of the VFA and reconstruction of the vertebrobasilar system. The patient remained clinically well. An endovascular approach using PEDs can be a safe and effective treatment option for ruptured VFA in selected cases.
Vertebrobasilar fusiform aneurysm (VFA), also known as vertebrobasilar dolichoectasia, is a vasculopathy characterized by pathologically elongated and dilated vertebral and basilar arteries with estimated incidence of 0.06 - 5.8%.10) It can be diagnosed radiographically using Smoker's Criteria.8) Clinical manifestations of VFA may include ischemic stroke, cranial nerve palsies, brainstem compression, hydrocephalus and intracranial hemorrhage.6)10) Subarachnoid hemorrhage (SAH) is an uncommon presentation of VFA with a crude incidence rate of 2.2 per 1000-person years.6) Traditional treatment options for ruptured VFAs have been limited and often carry significant mortality and morbidity.
The Pipeline embolization device (PED) (Covidien, CA) is a braided, self-expandable, platinum and nickel-cobalt chromium alloy wire mesh initially approved by Food & Drug Administration (FDA) for treatment of aneurysms in the internal carotid artery from the petrous to the superior hypophyseal segments. PED is considered a flow diverter. Since its FDA approval, PED has gained much popularity in an attempt to reconstruct vessels with abnormal morphology. Several centers have reported using PED for treating complex posterior circulation aneurysms with mixed results.1)5)7)9) In this report, we describe the use of PEDs to successfully treat a ruptured VFA.
A 73-year-old man presented with Hunt and Hess II SAH. There was no radiographic or clinical evidence of hydrocephalus on admission. He was on aspirin 325 mg and clopidogrel 75 mg daily for his coronary artery stent placed seven days earlier. Computed tomography (CT) angiography of the brain showed severely ectatic left vertebral and basilar arteries consistent with VFA. The right vertebral artery appeared irregular and non-dominant (Fig. 1).
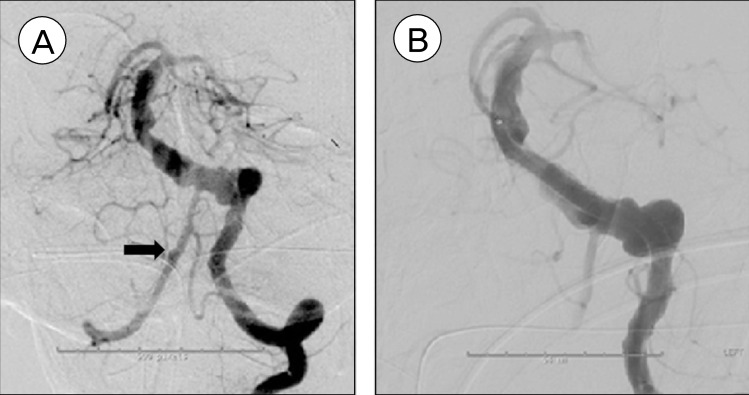
After careful evaluation, endovascular approach was deemed to be the most appropriate treatment. The patient was taken to neurointerventional suite and placed under general anesthesia. A complete 6-vessel cerebral angiogram demonstrated severe dolichoectasia involving the left vertebral and the basilar arteries. It also revealed a 2-mm blister in the right vertebral artery proximal to right PICA take off that was not apparent on pre-operative CT angiogram. Since either the left dolichoectatic vertebrobasilar artery or the right vertebral artery blister aneurysm could be the source of SAH, the decision was made to treat both.
Through a 6 French sheath, a 6F neuron guide catheter (Penumbra, CA) was used to catheterize the left vertebral artery. The patient was given 3,000 units of heparin intravenous injection with an activated clotting time of 214. His aspirin reaction units was 407; clopidogrel activity test showed 73% platelet inhibition with P2Y12 reactivity unit of 98. A Marksman catheter (Covidien, CA) along with a Synchro 2 (Stryker, MI) standard microwire was navigated through the left dolichoectatic vertebral artery into the basilar artery. Next, five PEDs (four 4.5 × 20 mm, one 4.5 × 18 mm) were deployed in a telescoping fashion starting from proximal part of the basilar artery down to the left vertebral artery, essentially reconstructing the entire length of the affected vertebrobasilar system. Each PED was placed within the previous one with 50% overlap. A cerebral angiogram after the PED placement demonstrated good patency of the target vessels and there was stasis in the irregularity outside the PED. Next, the right vertebral artery was selectively catheterized and one additional PED (3.0 × 16 mm) was placed across the blister in the right vertebral artery. The final angiogram from the left vertebral artery demonstrated patency of both vertebral arteries (Fig. 2). No technical complication occurred during the case. The patient was continued on aspirin 325 mg and clopidogrel 75 mg daily.
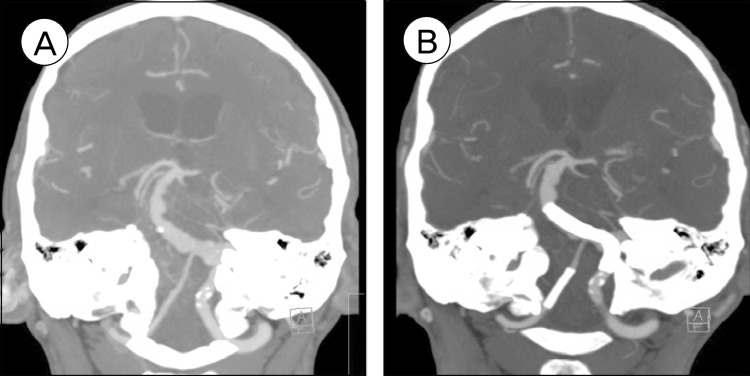
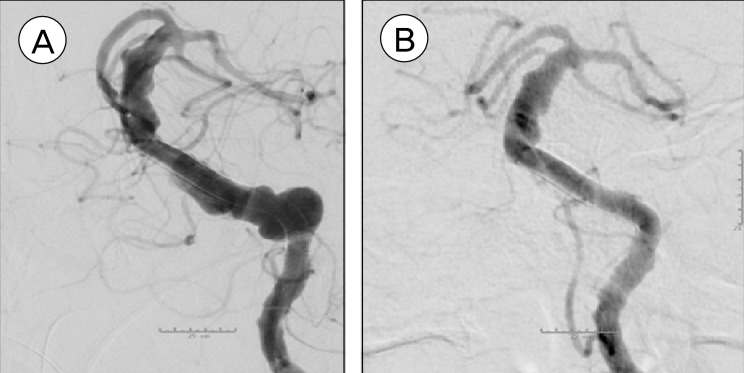
CT angiogram on postoperative day 5 showed patency of the stents (Fig. 3). MRI at three months after the procedure showed no ischemic changes suggesting patency of perforators in the vertebrobasilar system. Conventional cerebral angiogram at six months demonstrated successful reconstruction of the vertebrobasilar system and thrombosis of the VFA (Fig. 4). The patient remained neurologically well and was living independently at home.
SAH is an uncommon presentation of VFA and only accounts for about 3.85% of the cases overall.6) However, the risk for re-hemorrhage in these patients is high.4) Open surgical treatment in the setting of SAH in this elderly patient on aspirin and clopidogrel carries high risk. The optimal strategy and treatment goal in this setting would be strengthen and reconstruct the VFA to prevent recurrent hemorrhage and further comprise in vessel wall integrity. The PED is an ideal device in this situation. It provides a metal scaffold for endothelial cells to promote neointimal growth,1-3) which eventually forms an entirely new endothelial-lined lumen with metal reinforced vessel wall. In theory, this would decrease the chance for recurrent hemorrhage and may even deter or halt the disease progression. We recommend not overlapping PED's more than 50% of their length in the posterior circulation to avoid mechanical obstruction of perforators. Ideally, a longer length PED would have been our first choice to allow single device coverage of this aneurysm, but unfortunately, this device is not currently available in the United States.
The drawback of using a stent in the case of acute SAH is that the patient was continued on dual antiplatelet therapy, which might have complicated the hospital course if the patient would have needed external ventricular drainage or a shunting procedure. In addition, the use of PED in the acute phase of SAH is controversial due to the fact that PED might not immediately occlude the area that bled. Other endovascular options would have been sacrifice of the dominant vertebral artery with the hopes that the non-dominant vertebral artery would have sufficient perfusion. However, in this case the non-dominant vertebral artery was diseased as well. The use of regular self-expanding stents would have been an option, but would not have significant flow diversion.
References
1. Fiorella D, Kelly ME, Albuquerque FC, Nelson PK. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009; 2. 64(2):212–217. discussion 217. PMID: 19057425.

2. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009; 6. 30(6):1153–1158. PMID: 19369609.

3. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 8. 38(8):2346–2352. PMID: 17615366.

4. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; 5. 36(5):905–911. discussion 912-3. PMID: 7791980.

5. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the in tracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 1. 32(1):34–40. PMID: 21148256.
6. Passero SG, Calchetti B, Bartalini S. Intracranial bleeding in patients with vertebrobasilar dolichoectasia. Stroke. 2005; 7. 36(7):1421–1425. PMID: 15976311.

7. Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, et al. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg. 2012; 6. 116(6):1258–1266. PMID: 22404673.

8. Smoker WR, Corbett JJ, Gentry LR, Keyes WD, Price MJ, McKusker S. High-resolution computed tomography of the basilar artery: 2. Vertebrobasilar dolichoectasia: clinical-pathologic correlation and review. AJNR Am J Neuroradiol. 1986; Jan-Feb. 7(1):61–72. PMID: 3082145.
9. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010; 6. 31(6):1139–1147. PMID: 20150304.

10. Titlic M, Tonkic A, Jukic I, Kolic K, Dolic K. Clinical manifestations of vertebrobasilar dolichoectasia. Bratisl Lek Listy. 2008; 109(11):528–530. PMID: 19205567.
Fig. 1
Computed tomography (CT) of the brain without contrast (left) showing subarachnoid hemorrhage in the prepontine, ambient and crural cisterns; CT angiogram of the brain (right) showing a vertebrobasilar fusiform aneurysm, also known as vertebrobasilar dolichoectasia involving the left vertebral and basilar arteries. The right vertebral artery is irregular and non-dominant.
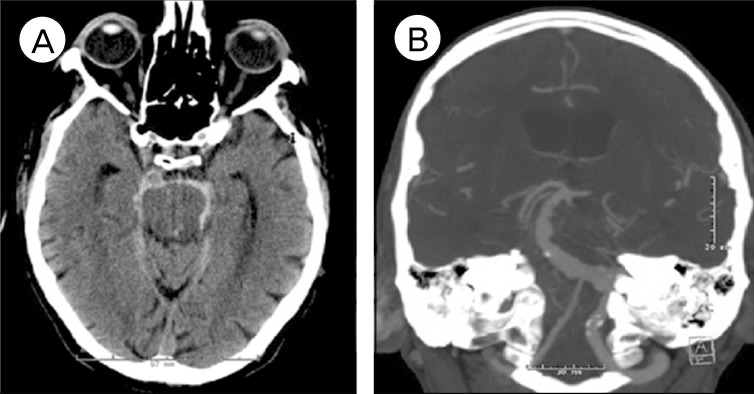
Fig. 2
Pretreatment conventional cerebral angiogram (left) demonstrating fusiform dilation of the left vertebral and basilar arteries, along with a 2 mm blister aneurysm (arrow) in the right vertebral artery proximal to posterior inferior cerebellar artery takeoff; post-treatment angiogram (right) demonstrating reconstruction of affected vessel lumen and stasis of blood flow in the irregular portion of the vessels outside of Pipeline Embolization Device construct.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download