Abstract
More effective treatments in first, second, and third-line of metastatic non-small cell lung cancer (NSCLC) enable patients to live longer, with a better quality of life (QOL). Especially epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) contributed to this improvement. Gefitinib was compared with Docetaxel in four randomized trials, i.e., SIGN, Japanese V-1532, Korean ISTANA, and INTEREST in second or third-line treatment of metastatic NSCLC. In all the trials, and also by meta-analysis of 2,257 patients in these trials, Gefitinib was found non-inferior or superior to Docetaxel, with less toxicity, convenient oral administration, and better QOL. Detailed results are presented in the review article. Knowing that every line of treatment we may lose about 50% of patients for further treatment, it is very important to offer each patient the best option for every line of treatment. Gefitinib has a favorable benefit-risk profile compared with Docetaxel in this patient population.
References
1. Schiller JH. Small cell lung cancer: defining a role for emerging platinum drugs. Oncology. 2002; 63:105–114.

2. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced nonsmall-cell lung cancer. N Engl J Med. 2002; 346:92–98.

3. Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, Socinski MA. Third-generation chemotherapy agents in the treatment of advanced nonsmall cell lung cancer: a metaanalysis. J Thorac Oncol. 2007; 2:845–853.

4. Abratt RP, Hart GJ. 10-year update on chemotherapy for nonsmall cell lung cancer. Ann Oncol. 2006; 17(Suppl 5):v33–36.

5. Stinchcombe TE, Socinski MA. Treatment paradigms for advanced stage nonsmall cell lung cancer in the era of multiple lines of therapy. J Thorac Oncol. 2009; 4:243–250.

6. Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with nonsmall-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103.

7. Fossella FV. Second-line chemotherapy for nonsmall-cell lung cancer. Curr Oncol Rep. 2000; 2:96–101.

8. Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with nonsmall-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–1597.

9. Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009; 14:253–263.

10. Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997; 57:4838–4848.
11. Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999; 291:739–748.
12. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005; 353:123–132.

13. Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006; 24:3831–3837.

14. Reck M, Mali P, Arrieta O, et al. Global efficacy and safety results from the trust study of erlotinib monotherapy in >7,000 patients with non small cell lung cancer (NSCLC). Ann Oncol. 2008; 19(Suppl 8):viii100.
15. Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced nonsmall-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–1537.

16. Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) nonsmall-cell lung cancer. Anticancer Drugs. 2006; 17:401–409.

17. Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15–32, of gefitinib versus docetaxel in previously treated Japanese patients with nonsmall-cell lung cancer. J Clin Oncol. 2008; 26:4244–4252.

18. Lee D, Kim S, Park K, et al. A randomized open-label study of gefitinib versus docetaxel in patients with advanced/me-tastatic nonsmall cell lung cancer (NSCLC) who have previously received platinum-based chemotherapy. Proc Am Soc Clin Oncol. 2008; 26:A8025.

19. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated nonsmall-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008; 372:1809–1818.

20. Douillard JY, Hirsh V, Mok T, et al. Molecular predictors of outcome with Gefitinib and Docetaxel in previously treated non small cell lung cancer: data from the randomized phase III INTEREST Trial. J Clin Oncol. Forthcoming.
21. Shepherd F, Douillard JY, Fukuoka M, et al. Gefitinib versus Docetaxel in patients with pretreated advanced non small cell lung cancer (NSCLC): metaanalysis from four randomized clinical trials. J Thorac Oncol. 2009; 4(9 Suppl 1):S291.
Figures and Tables
Fig. 1.
SIGN: quality of life (QOL) & symptom improvement, LCS: lung cancer subscale, FACT-L: functional assessment of cancer therapy-lung, CI: confidence interval.
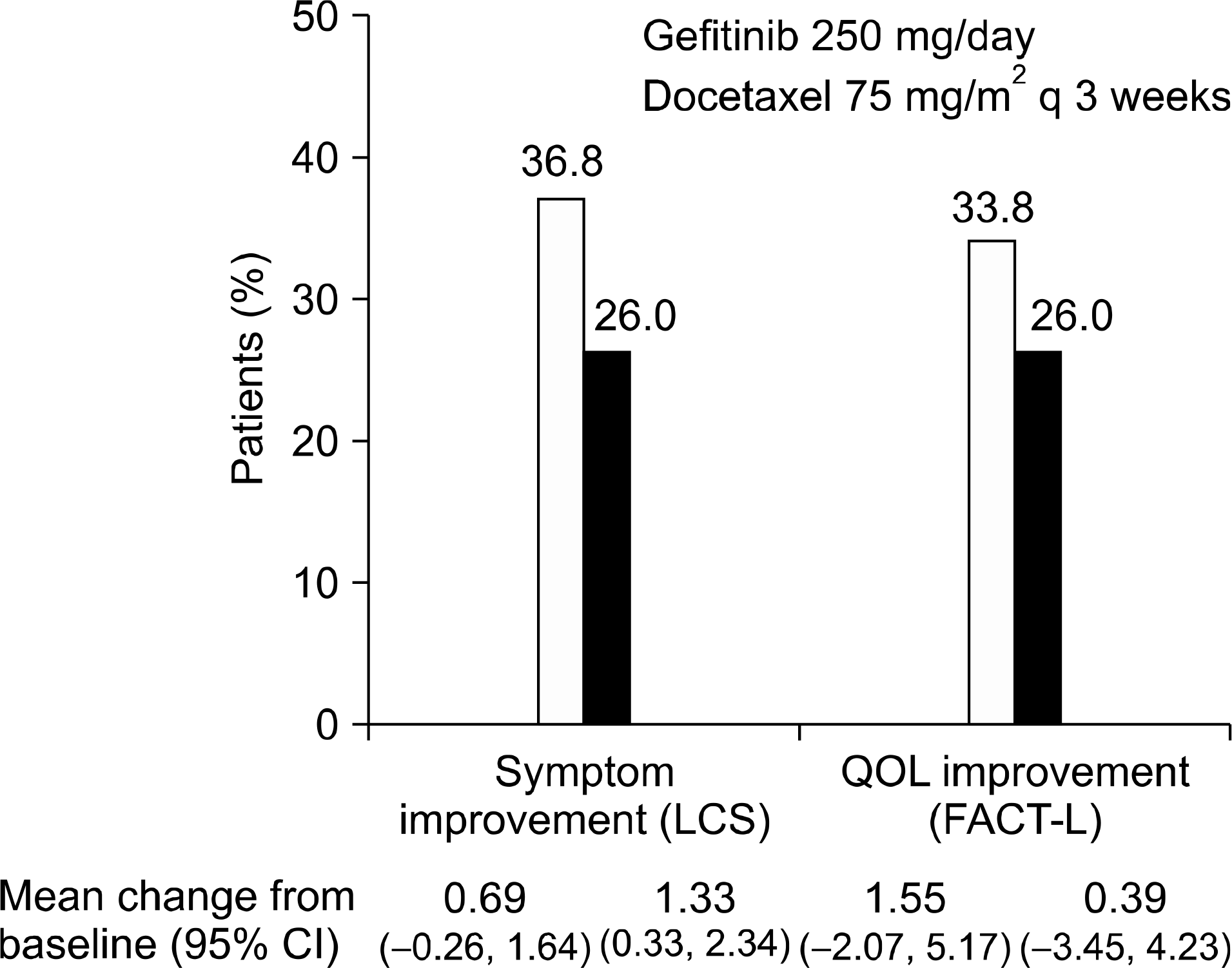
Fig. 2.
Quality of life and symptom improvement rates (EFQ population) – INTEREST. p values from logistic regression with covariates. Clinically relevant improvement pre-defined as 6-point improvement for FACT-L and TOI; 2-point improvement for LCS, maintained for at least 21 days. EFQ: evaluable for quality of life, FACT-L: functional assessment of cancer therapy-lung, TOI: trial outcome index, LCS: lung cancer subscale.
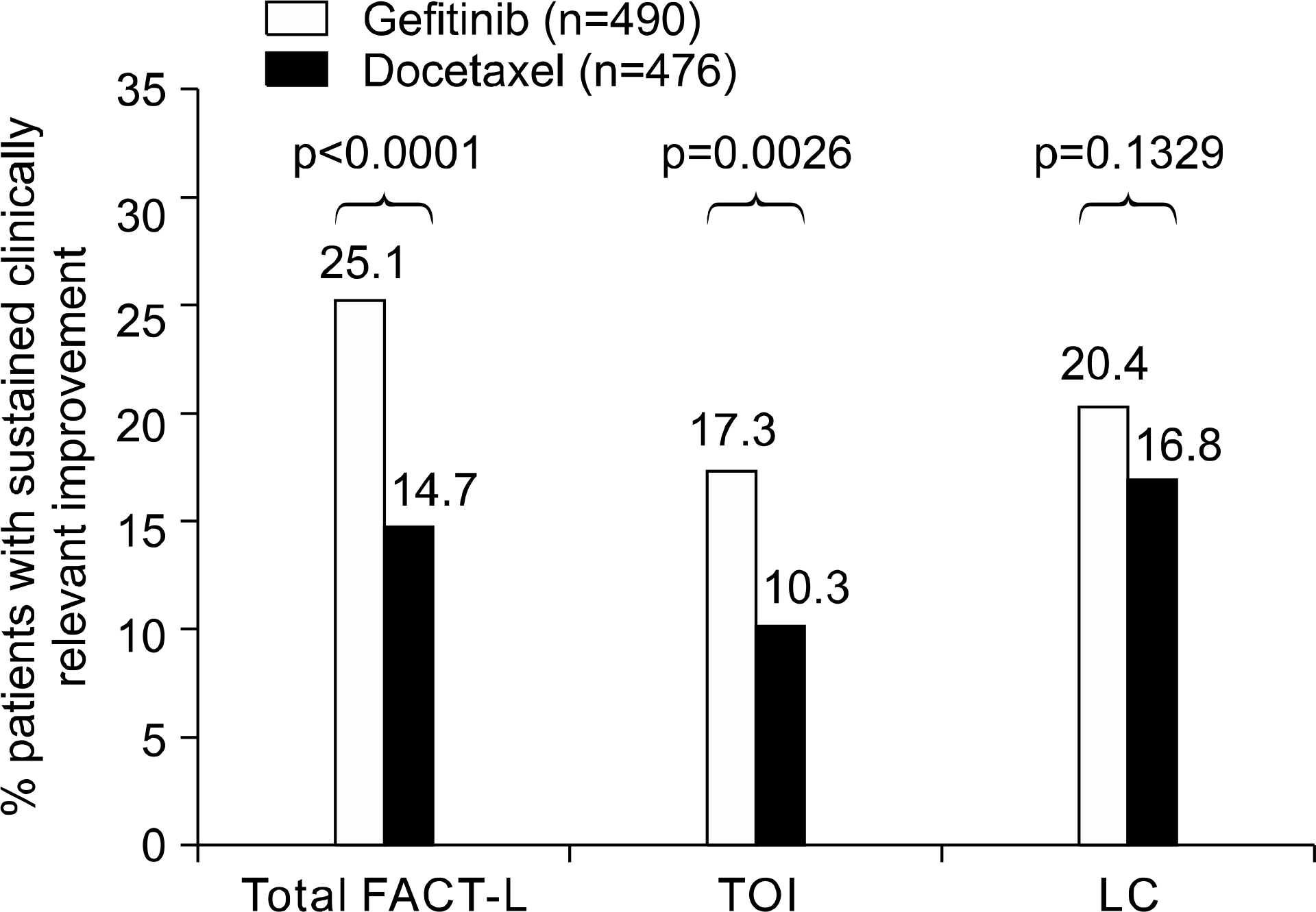
Fig. 3.
Kaplan-Meier curves of (A) overall survival and (B) progression-free survival for all patients (Meta-analysis).
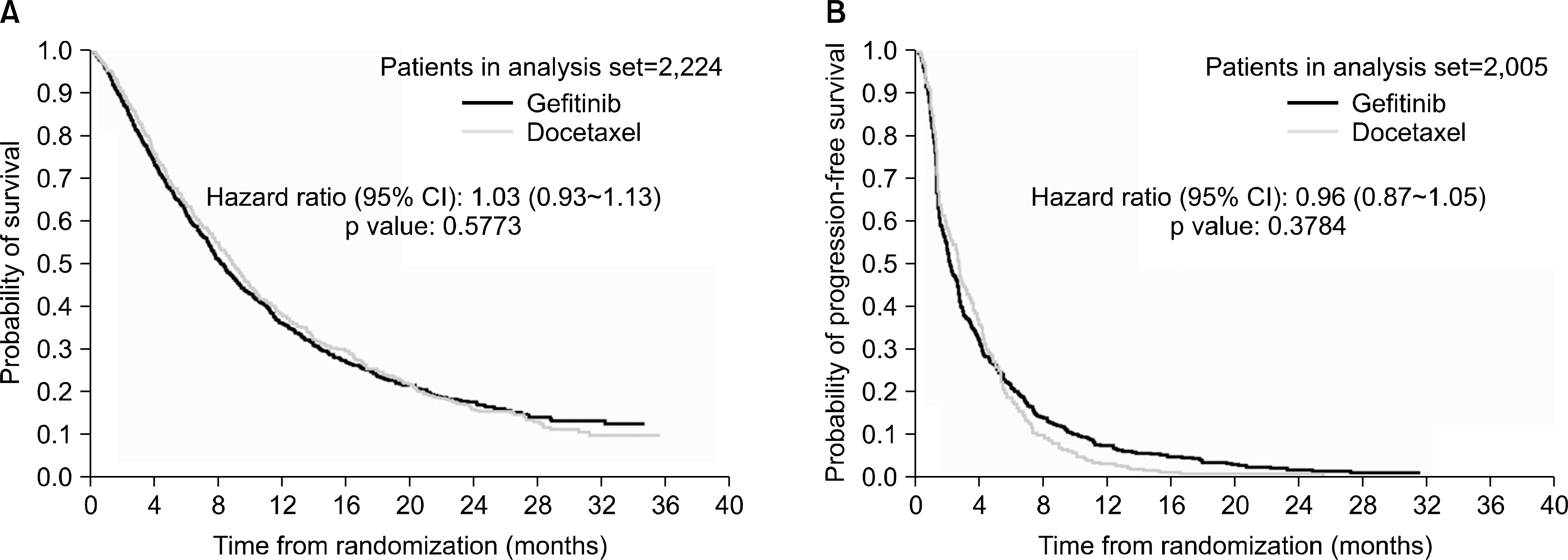
Table 1.
Efficacy Data in the Second-Line Setting
Table 2.
Demography (ITT Population) – INTEREST
| Gefitinib, % (n=733) | Docetaxel, % (n=733) | |
|---|---|---|
| Age <65 years | 61 | 67 |
| Female | 36 | 33 |
| WHO PS 0/1/2* | 30/58/12 | 25/63/12 |
| Never-smoker* | 20 | 20 |
| Second-line* | 84 | 83 |
| Asian origin | 21 | 23 |
| Adenocarcinoma* | 54 | 55 |
| Since diagnosis: | 26/38/35 | 27/37/35 |
| <6/6∼12/>12 months | ||
| Prior platinum | 54/45 | 56/42 |
| refractory†/received* | ||
| Prior paclitaxel | 9/9/81 | 8/9/82 |
| refractory†/received/none* | ||
| Best response to previous | 27/41/26 | 31/38/25 |
| CT (CR+ PR)/SD/PD | ||
| Locally advanced disease | 14 | 13 |
Table 3.
Phase III Gefitinib vs. Taxotere: Overall Survival (INTEREST)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download