Abstract
Liriope platyphylla (LP) has long been regarded as a curative herb for the treatment of diabetes, asthma, and neurodegenerative disorders. To examine the therapeutic effects of Red LP (RLP) manufactured by steaming process on neurodegenerative disorders, significant alteration of the key factors influencing Alzheimer's Disease (AD) was detected in NSE/hAPPsw transgenic (Tg) mice after RLP treatment. The concentration of nerve growth factor (NGF) in serum increased in RLP-treated NSE/hAPPsw Tg mice compared with vehicle-treated Tg mice. However, downstream effectors of the NGF receptor signaling pathway, including TrkA and p75NTR proteins, were suppressed in RLP-treated NSE/hAPPsw Tg mice. Especially, Tg mice showed decreased levels of TrkA, p75NTR, and RhoA expression. Production of Aβ-42 peptides was lower in RLP-treated NSE/hAPPsw Tg mice than in vehicle-treated Tg mice. Further, analysis of γ-secretase components showed that Aβ-42 peptide expression was downregulated. Of the four components, the expression of APH-1 and Nicastrin (NCT) decreased in RLP-treated NSE/hAPPsw Tg mice, whereas expression of PS-2 and Pen-2 was maintained or increased within the same group. Overall, these results suggest that RLP can help relieve neurodegenerative diseases, especially AD, through upregulation of NGF secretion ability, activation of NGF signaling pathway, downregulation of Aβ-42 peptide deposition, and alteration of γ-secretase components.
LP has been widely used for the treatment of various chronic diseases, including asthma as well as bronchial and lung inflammation, in Korea for a long time [1]. Many studies have recently demonstrated that extracts of LP roots effectively prevent obesity, diabetes, inflammation, and neurodegenerative disease [2-7]. Among these therapeutic effects, LP also exhibits therapeutic potential in human subjects suffering from neurodegenerative disorders such as AD. In particular, the steroidal saponin spicatoside A, isolated from LP, induces neuritic outgrowth similar to NGF and activates extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3-kinase/Akt) in PC12 cells [7]. Among the two types of neuronal cells, B35 and C6, it has been shown that 10% water extract of LP induces an increase in NGF secretion, PC12 cell differentiation, and NGF mRNA expression [8].
Steaming, a common manufacturing technique, is often applied to medicinal herbs to enhance the concentration or efficacy of functional components and induce chemical transformations of therapeutic components [9]. This process is applied most successfully to ginseng plant, derivations of which are taken orally as adaptogens, aphrodisiacs, and nourishing stimulants, as well as in the treatment of type II diabetes and sexual dysfunction in men [10-12]. There are two types of Korean ginseng: Korean white ginseng (KWG) (Panax ginseng C.A. Meyer), which is air-dried ginseng, and Korean red ginseng (KRG) (Ginseng Radix Rubra), which is steamed [9]. During the steaming-process of ginseng, several important components, including ginsenosides, acidic polysaccharides, and phenolics, are transformed into different components, and several new compounds, such as non-saponinpolyacetylene, maltol, and amino acids, are formed [13,14]. Recently, this process was applied to LP to increase its effects on insulin secretion ability and the insulin receptor signaling pathway. Maximum insulin secretion was observed in INS cells treated with LP extract steamed for 3 h with two repeated steps (3 h steaming and 24 air-dried) carried out 3 times (3-SALP) and 9-SALP [15]. Despite these primary results, there has not been confirmed whether or not RLP is able to alter the pathological phenotypes of neurodegerative disorders such as AD observed in NSE/hAPPsw Tg mice.
Therefore, this study investigated the effects of RLP on neurodegenrative disorder-related factors, including NGF secretion ability, NGF receptor signaling pathway, Aβ-42 production, and γ-secretase expression, as well as the potential of RLP as a therapeutic drug applied to neuronal-related diseases. These results provide a scientific basis for determining the optimal conditions for the LP steaming process when applied to neuronal-related diseases.
Roots of LP were collected from plantations in the Miryang area (Korea) and dried in a hot-air drying machine (JSR, Seoul, Korea) at 60℃. To prepare extract at seven different steaming frequencies, the specific process comprising two steps (200 g of dry roots was steamed at 99℃ for 3 h and air-dried at 70℃ for 24 h) was carried out for different numbers of repetitions a total of seven times. The steamed roots were reduced to powder using an electric blender. The water extracts were purified for 2 h at 100℃ using circulating extraction equipment (IKA Labortechnik, Staufen, Germany) after adding 200 mL of distilled water. In addition, a solution of the extracts was concentrated to dry pellets in a rotary evaporator (EYELA, Tokyo, Japan) and stored at -80℃ until needed. The final yield of RLP has been calculated about 86.22%.
The animal protocol used in this study was reviewed and approved based on ethical procedures and scientific care by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2011-00220). Adult NSE/hAPPsw Tg mice were obtained from Korea FDA and handled at the Pusan National University Laboratory Animal Resources Center according to National Institutes of Health guidelines. All mice were given a standard irradiated chow diet (Purina Mills Inc., Seoungnam, Korea) ad libitum and were maintained in a specific pathogen-free state under a strict light cycle (light on at 06:00 h and off at 18:00 h) at a temperature of 22±2℃ and at 50% relative humidity.
NSE/hAPP Tg mice used in this study were produced by microinjection of hAPP cDNA under control of the neuron-specific enolase (NSE) promoter in a previous study (Figure 1A) [16]. At 12 months of age, they showed pathological phenotypes, including behavioral dysfunction, Aβ-42 overproduction, tau hyperphosphorylation, and increased apoptosis.
Twelve-month-old Tg mice (n=10) were assigned to one of the following two groups: vehicle-treated group and RLP-treated group. The first group of NSE/hAPPsw Tg mice received a comparable volume of daily water via gavage (vehicle-treated group), whereas the second group received 50 mg/kg body weight/day of RLP via gavage (RLP-treated group). At 3 weeks after RLP treatment, all animals were immediately sacrificed using CO2 gas for preparation of blood and tissue samples, which were stored in Eppendorf tubes at -70℃ until assayed.
To identify NSE/hAPPsw Tg mice, genotyping via polymerase chain reaction (PCR) analysis was performed on genomic DNA isolated from mouse tails. For DNA-PCR, 10 pmol each of NSE-specific primers, sense: 5'-CTG AGT CTG CAG TCC TCG A-3' and APP-specific primers, antisense: 5'-CTC TTC TCA CTG CAT GTC TC-3' (Figure 1A) were added into genomic DNA template mixtures, and the reaction mixtures were subjected to 25 cycles of amplification. Amplification was conducted in a thermal cycler T100 (Bio-Rad Laboratories Inc., Hercules, California, USA) under the following conditions: denaturation for 30 sec at 95℃, annealing for 30 sec at 62℃, and extension for 45 sec at 72℃. The amplified PCR products were loaded on a 1.0% agarose gel, after which the bands were detected using the Kodak Electrophoresis Documentation and Analysis System 120 (Eastman Kodak, Rochester, NY, USA).
The levels of NGF in the sera collected from vehicle- and RLP-treated Tg mice were measured by following the ultra-sensitive assay of the NGF ELISA kit (Chemicon International Inc., CA, USA). Briefly, the sample and standards were incubated overnight on antibody-coated plates in a plate shaker at 100-150 rpm at 2-8℃. The wells were then washed four times with washing buffer, after which 100 µL of anti-mouse NGF monoclonal antibody was added to each of the wells. Plates were then incubated in a shaker for 2 h at room temperature. The next step involved adding 100 µL of peroxidase-conjugated donkey anti-mouse IgG polyclonal antibody to each well, followed by incubation at room temperature for 2 h. After washing, 100 µL of TMB/E substrate was added to each well and the plate incubated at room temperature for 15 min. The reaction was quenched by the addition of 100 mL of stop solution. The plates were analyzed by evaluating the absorbance at 450 nm using an ELISA reader (VERSA max, micro-reader, MDS Co., USA).
Proteins prepared from the brain tissues of vehicle- and RLP-treated Tg mice were separated by electrophoresis on a 10-20% SDS-PAGE gel for 2 h and then transferred to nitrocellulose membranes for 2 h at 40V. Each membrane was incubated separately with a primary antibody: anti-TrkA antibody (Cell Signaling Technology, Beverley, MA, USA), anti-p-TrkA antibody (Cell Signaling Technology), anti-Akt antibody (Cell Signaling Technology), anti-p-Akt antibody (Cell Signaling Technology), anti-ERK antibody (Santa Cruz Biotechnology, Santacruz, CA, USA), anti-p-ERK antibody (Santa Cruz Biotechnology), anti-p75NTR antibody (Cell Signaling Technology), anti-RhoA antibody (Cell Signaling Technology), anti-Bax antibody (Abcam, Cambridge, UK), anti-Bcl-2 (Abcam), anti-Caspase-3 (Abcam) anti-PS-2 antibody (Cell Signaling Technology), anti-APH-1 antibody (Sigma-Aldrich), anti-NCT antibody (Cell Signaling Technology), anti-Pen-2 antibody (Santa Cruz Biotechnology), or anti-beta actin (Sigma-Aldrich). Each membrane was then washed with buffer (137 mM NaCl, 2.7 mM KCl, 10 mM NaHPO4, and 0.05% Tween-20) and incubated with a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG at room temperature for 2 h. The membrane blots were developed using an Enhanced Chemiluminescence (ECL) Reagent Plus kit (Amersham Life Science, Piscatway, NJ, USA).
Brain perfusion and immunohistochemical analyses were performed as previously described [17,18]. Briefly, mice were anaesthetized with CO2 gas and transcardially perfused with 1× PBS followed by 4% formaldehyde in order to effectively remove blood and fix brain tissue. After perfusion, each mouse brain was isolated from the skull and fixed overnight in formaldehyde. Each brain was then dehydrated and embedded in paraffin. A series of brain sections (10 µm) were cut from paraffin-embedded tissue using a Leica microtome (Leica Microsystems, Bannockbrun, IL, USA). For immunohistochemical analysis, these sections were de-paraffinized with xylene, rehydrated, and pretreated for 30 min at room temperature with PBS blocking buffer containing 10% goat serum. The sections were then incubated with anti-Aβ-42 antibody (Invitrogen, Corporation, CA, USA), at a dilution of 1:100 in PBS blocking buffer. The antigen-antibody complexes were visualized using biotinylated secondary antibody (goat anti-rabbit)-conjugated HRP streptavidin (Histostain-Plus Kit; Zymed, South San Francisco, CA, USA) at a dilution of 1:1,500 in PBS blocking buffer. Aβ-42 peptides were detected using stable 3,3'-diaminobenzidine (DAB; Invitrogen) and observed using a model BX50F-3 optical microscope (Olympus, Tokyo, Japan).
Proteins prepared from brains of vehicle- and RLP-treated mice were transferred to a nitrocellulose membrane using a Slot Blot kit (Pharmacia Biotech, CA, USA). The membrane was incubated separately with primary rabbit polyclonal anti-Aβ-42 antibody at room temperature for 1 h, followed by incubation with secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG (GenTest, MA) at 1:1,000 for 1 h at room temperature. Aβ-42 specific peptide was detected using an ECL Reagent Plus kit. Anti-Aβ-42 was able to specifically detect Aβ-42 on the slot blot as recommended by the manufacturer.
The levels of soluble Aβ-42 from brains of vehicle- and RLP-treated mice were measured by following the ultra-sensitive assay procedure of the Human Aβ-42 ELISA kit (Invitrogen). The frontal lobe from the brain of each mouse was homogenized in 10 volumes of guanidine-tris buffer (5.0 M guanidine HCl/50 mM Tris-HCl, pH 8.0). The homogenates were then mixed for 3 h at room temperature and stored at 20℃ until analyzed [19,20]. An Aβ-42 ELISA kit was used to measure the level of Aβ-42 in the brain homogenates according to the manufacturer's instructions. In brief, the sample or standards along with Human Aβ-42 detection antibody solution were incubated on antibody-coated plates. Wells were then washed three times, after which HRP conjugate was added to each of the wells for 30 min. The reaction was terminated via the addition of 50 µL of stop solution, after which the plates were analyzed by evaluating the absorbance at 450 nm using a Molecular Devices Vmax Plate Reader (Sunnyvale, CA, USA).
Tests for significance between the various types of vehicle- and RLP-treated groups were carried out using One-Way ANOVA test of variance (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). All values are reported as the mean±standard deviation (SD). A P value of <0.05 was considered significant.
For this test, the genotypes of NSE/hAPPsw Tg mice were firstly identified via PCR analysis using genomic DNA isolated from the tails of 3-week-old mice. After electrophoresis, the PCR products of the NSE/hAPPsw construct were detected on 1.0% agarose gels as 512-bp bands (Figure 1A). All mice used in this study were identified as NSE/hAPPsw Tg mice.
To ascertain whether or not RLP treatment affects regulation of NGF secretion in NSE/hAPPsw Tg mice, serum concentrations of NGF were measured using an ELISA kit. The Tg mice treated with RLP showed 150% higher NGF concentrations than Tg mice treated with vehicle alone (Figure 1B). These results suggest that RLP treatment increased NGF secretion in the serum of NSE/hAPPsw Tg mice.
This study examined the effects of NGF induced by RLP on the NGF receptor TrkA and p75NTR signaling pathways in brain tissue via Western blot analysis. First, in an analysis of the high affinity receptor, phosphorylation of TrkA significantly decreased in the RLP-treated group due to enhanced TrkA expression. Next, phosphorylation of Akt and Erk, which is activated by transferred TrkA, was examined. There was no change in Akt protein phosphorylation, whereas a significant change was detected in phosphorylation of ERK protein. The level of Erk phosphorylation increased about 20% in the RLP-treated group (Figure 2A).
On the other hand, the level of p75NTR expression was lower in the RLP-treated group than the vehicle-treated group. Moreover, the level of RhoA, a downstream signal of p75NTR, was lower in the RLP-treated group (Figure 2B). These patterns of expression were further observed in the apoptosis-related proteins Bcl-2, Bax and casepase-3. The ratio of Bcl-2/Bax expression was lower in the RLP-treated group, whereas caspase-3 expression was maintained (Figure 2C). These results show that RLP induced significant changes in the NGF receptor TrkA and p75NTR signaling pathways.
We investigated whether or not accumulation of Aβ-42 peptides could be improved by RLP treatment. To achieve this, the concentration of Aβ-42 peptides was measured in the brain cortex of NSE/hAPPsw Tg mice after 3 weeks of treatment. According to the results of the immunohistochemical analysis and dot blot assay, the Aβ-42 concentration was significantly reduced in the RLP-treated group compared to vehicle-treated group (Figure 3A and B). Further, significant alteration of the concentration of soluble Aβ-42 was detected between the vehicle- and RLP-treated groups (Figure 3C). These results suggest that RLP induced a decrease in Aβ-42 concentration in NSE/hAPPsw Tg mice.
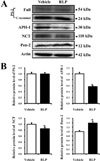
To investigate the effects of RLP on the expression of γ-secretase components, the levels of these four proteins were measured in the brain cortex of NSE/hAPPsw Tg mice. There was no change in the expression level of full or C-terminal PS-2. However, APH-1 and NCT expression significantly decreased in the RLP-treated group compared to vehicle-treated group, whereas Pen-2 expression slightly increased (Figure 4). These results indicate that expression of γ-secretase components in the brain cortex of NSE/hAPPsw Tg mice was controlled by RLP administration.
NSE/hAPPsw Tg mice were previously produced by microinjection of the NSE/hAPPsw transgene into the male pronucleus of fertilized embryos in our previous study [16]. These mice showed behavioral dysfunction, increased APP expression, and production of Aβ-42 at 12 months. Further, both JNK and p38 were activated in the brains of NSE/hAPPsw Tg mice, whereas there was no significant activation of ERK. Moreover, significant alteration of Cox-2 levels along with caspase-3- and TUNEL-stained nuclei were detected in brains of the transgenic line [16]. Therefore, in this study, we chose the model best suited for determining whether or not RLP administration could relieve the pathological phenotypes of neurodegenerative disorders. Although our study has provided significant results on RLP efficacy, more research is needed using other animal models of AD.
Few studies have been conducted regarding on the effect of traditional medicine on neurodegenerative disorders using the Tg2576 mouse model for AD. Treatment with 1.0% yokukansan (TJ-54), a traditional Japanese medicine, has been shown to ameliorate learning deficits and non-cognitive defects, including anxiety, as well as increase locomotor activity in Tg2576 mice [27]. In another study, pre-treatment with aged garlic extract (AGE) protected 80% neuronal cells from ROS-mediated damage. Furthermore, treatment with 2% AGE-containing diet and S-ally-L-cystein (SAC) independently increased the concentrations of synaptosomal-associated protein of 25-kDa (SNAP25) and synaptophysin in Tg2576 mice [28]. Also, our previous study observed that maximum NGF secretion from neuronal B35 cells occurs upon treatment with 50 µg/mL of 7-SALP [15]. In this study, the functions of RLP in neuronal cells were verified using NSE/hAPPsw Tg animal model. NGF ELISA analysis showed that RLP was effectively worked even in Alzheimer's model animal overexpressing APPsw protein.
Secreted NGF transduced the signal into the cytosol by binding two types of NGF receptors located on the cell membrane. Of the two types of receptors, a high affinity receptor, TrkA, can induce cell survival through Akt as well as neuritic outgrowth through the ERK signaling pathway. Further, a low affinity NGF receptor, p75NTR, has additional independent functions, including a role in pro-apoptotic signaling [21-24]. As shown Figure 2, the NGF secreted by RLP treatment induced the activation of cytosolic signaling pathway through two types of NGF receptor. These results showed the possibility that RLP may consider as potential stimulator for NGF receptor activation.
In addition, the accumulation of Aβ-42 peptides is an important indicator of AD [25,26]. These peptides are produced from APP by cleavage of γ-secretase, which consists of PS, NCT, APH-1 and Pen-2 [25]. Our results showed that RLP could induce the decrease of Aβ-42 peptides production through the regulation of γ-secretase expression. However, this study did not suggest the correlation between NGF and Aβ-42 peptides production or between NGF and γ-secretase expression. Therefore, the further studies were needed to identify the detail mechanism on therapeutic action of RLP.
Taken together, our results demonstrate the effect of RLP on NGF secretion ability, NGF receptor signaling pathway, Aβ-42 production, and γ-secretase component expression in NSE/hAPPsw Tg mice. Moreover, RLP can be presently considered as a potential therapeutic candidate for neurodegenerative disorders.
Figures and Tables
Figure 1
Identification of Tg mice and NGF secretion. (A) pNSE/hAPPsw harbors the encoding hAPPsw gene under control of the NSE promoter. In the identification of Tg mice, PCR was performed on genomic DNA isolated from tails of founder mice, and the resulting products (512-bp) are shown. (B) NGF concentration in blood serum of NSE/hAPPsw Tg mice was measured using an anti-NGF ELISA kit. Data are reported as the mean±SD of three experiments. *P<0.05 is the significance level relative to vehicle-treated group.

Figure 2
Effects of RLP on downstream signaling pathway of NGF receptor, including (A) TrkA signaling pathway and (B) p75NTR signaling pathway. Total cell lysates were prepared from brain cortex of NSE/hAPPsw Tg mice treated with vehicle or RLP as described in the Materials and Methods. Fifty micrograms of protein per sample was immunoblotted with antibodies for each protein. Three samples were assayed in triplicate by Western blotting. Data are reported as the mean±SD. *P<0.05 is the significance level compared to cells treated with vehicle.
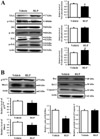
Figure 3
Effects of RLP on production of Aβ-42 peptides. Immunostaining analysis of Aβ-42 peptide accumulation. Production of Aβ-42 peptides in brains of 10-month-old NSE/hAPPsw Tg mice was detected by immunostaining (A), dot blot assay (B), and Aβ-42 ELISA assay (C). In immunostaining analysis, low intensity was observed in the hippocampus (CA1-3) and DG of RLP-treated NSE/hAPPsw Tg mice (Ae), as compared with vehicle-treated mice (Aa), at 200× magnification. Detailed histological features of several regions of the hippocampus are shown in three rectangles at 400× magnification (Ab-d and f-h). Left column of Aβ-42 ELISA assay shows the correlation between optical density at 450 nm and concentration of Aβ-42 peptides. Data are reported as the mean±SD. *P<0.05 is the significance level compared to mice treated with vehicle.
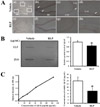
Figure 4
Expression of γ-secrease components in brain cortex of NSE/hAPPsw Tg mice. Cortex region was prepared from brain tissues of vehicle- and RLP-treated Tg mice. Fifty micrograms of protein per sample was immunoblotted with antibody for each protein. Expression levels of the four γ-secretase components were measured with specific antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG, as described in the Materials and Methods section. The intensity of each protein was calculated using an imaging densitometer. Data are reported as the mean±SD from three replicates. *P<0.05 is the significance level compared to the vehicle-treated group.
Acknowledgment
This study was supported by grants to Dr. Dae Youn Hwang from the Korea Institute of Planning Evaluation for Technology of Food, Agriculture, Forestry and Fisheries (110119-3).
References
1. Lee YC, Lee JC, Seo YB, Kook YB. Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. nhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J Ethnopharmacol. 2005. 101(1-3):144–152.
2. Choi SB, Wha JD, Park S. The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 2004. 75(22):2653–2664.
3. Jeong S, Chae K, Jung YS, Rho YH, Lee J, Ha J, Yoon KH, Kim GC, Oh KS, Shin SS, Yoon M. The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARalpha action in OLETF rats. J Ethnopharmacol. 2008. 119(2):245–251.
4. Kim SW, Chang IM, Oh KB. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by medicinal plants. Biosci Biotechnol Biochem. 2002. 66(12):2751–2754.
5. Lee YK, Kim JE, Nam SH, Goo JS, Choi SI, Choi YH, Bae CJ, Woo JM, Cho JS, Hwang DY. Differential regulation of the biosynthesis of glucose transporters by the PI3-K and MAPK pathways of insulin signaling by treatment with novel compounds from Liriope platyphylla. Int J Mol Med. 2011. 27(3):319–327.
6. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004. 27(8):1257–1260.
7. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009. 620(1-3):9–15.
8. Choi SI, Park JH, Her YK, Lee YK, Kim JE, Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. Effects of Water Extract of Liriope platyphylla on the mRNA Expression and Protein Secretion of Nerve Growth Factors. Korean J Med Crop Sci. 2010. 18:291–297.
9. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008. 120(2):190–195.
10. Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009. 7(3):293–302.
11. Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006. 58(8):1007–1019.
12. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003. 68(8):1539–1542.
13. Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996. 62(1):86–87.
14. Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996. 17(4):293–298.
15. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway. Lab Anim Res. 2011. 27(2):117–126.
16. Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002. 16(8):805–813.
17. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010. 1327:118–124.
18. Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004. 65(11):1328–1335.
19. Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, Zhou XF. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer's disease. Neurobiol Aging. 2009. 30(3):364–376.
20. Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994. 25(11):1373–1385.
21. Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, Kase Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer's disease. J Ethnopharmacol. 2009. 122(1):157–162.
22. Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011. 117(3):388–402.
23. Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001. 58(8):1045–1053.
24. Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001. 11(3):281–286.
25. Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999. 19(19):8207–8218.
26. Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004. 14(3):379–383.
27. Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004. 23(1-2):105–114.
28. Hwang DY, Cho JS, Lee SH, Chae KR, Lim HJ, Min SH, Seo SJ, Song YS, Song CW, Paik SG, Sheen YY, Kim YK. Aberrant expressions of pathogenic phenotype in Alzheimer's diseased transgenic mice carrying NSE-controlled APPsw. Exp Neurol. 2004. 186(1):20–32.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download