Abstract
Purpose
To date, standard semen parameters have been the only parameters investigated in sperm samples of infertile men in Algeria. We investigated, for the first time, semen parameters according to sperm DNA fragmentation (SDF) in these subjects.
Materials and Methods
SDF was determined by a validated sperm chromatin dispersion test in 26 infertile men. Patients were split into two groups according to the SDF level estimated by the DNA fragmentation index (DFI): the low fragmentation group (LFG; LFG with DFI ≤18%) and high fragmentation group (HFG; HFG with DFI >18%). The standard semen parameters were measured in both groups.
Results
We found that semen concentration and motility were negatively correlated with DFI (r=-0.65, r=-0.45, respectively; p<0.05), while morphology and semen volume were not correlated with it (r=0.24, r=-0.18, respectively; p>0.05). Comparison of the sperm concentration revealed that it was significantly higher in LFG than in HFG (37.57%±13.16% vs. 7.32%±3.59%, respectively; p<0.05), whereas no significant difference was observed regarding sperm motility and morphology.
Conclusions
Our findings suggest that SDF correlates well with both sperm motility and concentration but not with morphology. Thus, we conclude that SDF evaluation provides additional information regarding sperm quality, and should be used as a complementary test for assessing semen characteristics in infertile males.
Semen quality determination is of great importance for both infertility management for couples and for reproductive toxicology. Since 1980, the World Health Organization (WHO) has published five editions of the "Laboratory manual for the examination and processing of human semen", in order to achieve greater standardization of semen examination procedures and reference values [1], which were controversial before the latest edition of this manual, as authors from different centers found that the cut-off limits of sperm concentration, morphology, or motility were either excessively high or excessively low in the previous editions [2,3], leading to infertility over- or under-diagnosis. However, WHO criteria remain contentious even after the publication of the fifth edition. For example, sperm concentration cut-off limits, shifted from 20 million sperm/mL to the 5th centile of sperm density (i.e., 15 million sperm/mL), which resulted in some men who were considered infertile being reclassified as fertile [4].
Some authors have sought to overlap the problem of a unique threshold by setting two thresholds for every standard parameter (concentration, morphology, and motility) so that men can be classified as fertile, sub-fertile, or with intermediate fertility [5]. Some other researchers have sought other tests, in addition to those recommended in the WHO manuals, in order to better assess men's fertility. Among them are sperm function tests, oxidative stress tests or sperm chromatin, and DNA fragmentation tests [6]. The latter tests seem to be the ones most adopted in routine clinical practice. Indeed, DNA integrity is crucial to ensuring that the fertilizing spermatozoon (SPZ) can sustain normal embryonic development of the zygote [7] and correlates with reproductive success [8].
In this study, we used an sperm chromatin dispersion (SCD) test to evaluate sperm DNA fragmentation (SDF) in male partners of infertile couples, and explored the relationship of SDF with the WHO standard semen parameters. Geographic variation in semen quality has been observed [9,10], and this is the first evaluation of SDF in an Algerian population along with standard semen parameters.
Twenty-six male partners of candidate couples for intracytoplasmic sperm injection (ICSI) were involved in this prospective study at the Ibn Rushd Center of Reproductive Medicine of Constantine, Eastern Algeria. The necessary precautions were taken to protect the participants, according to the principles of the Declaration of Helsinki. Informed written consent was obtained from all of the patients.
The men's average age was 37.50±0.88 years and infertility dated back to at least one year prior (6.04±0.54 years). It was primary in 88.46% of cases and secondary in the remaining 11.54% cases. The etiology of infertility varied among oligospermia, asthenospermia, and teratospermia. The patients had no history of radiotherapy, chemotherapy, chronic illness, or varicocele. Only four smokers were recorded.
Semen samples were collected in sterile containers by masturbation after 3 to 5 days of sexual abstinence. After semen liquefaction at room temperature for at least one hour, density gradient centrifugation (DGC) was carried out. Briefly, 100 µL of PureSperm™ buffer (Nidacon International AB, Molndal, Sweden) were added to 900 µL of 100% PureSperm™ medium to obtain 1,000 µL of 90% PureSperm™. Five hundred fifty microliters of PureSperm™ buffer were added to 450 µL of 100% PureSperm™ medium to obtain 1,000 µL of 45% PureSperm™. One milliliter of the liquefied semen was then added on top of two layers of gradients (90% and 45%), the whole was centrifuged at 300 g for 25 minutes, and the supernatant was removed to add 200 µL of washing solution: FertiCult™ Flushing medium (FertiPro N.V, Beernem, Belgium). After centrifugation at 500 g for 10 minutes, the washing solution was aspirated and a culture medium (FertiCult™ IVF medium) was added to the pellet. An aliquot of unprocessed semen was kept in order to be freshly used for SCD assessment in a subsequent stage.
Standard semen parameters (volume, concentration, motility, and morphology) were evaluated according to the WHO guidelines [11].
The concentration was assessed by examining samples using phase-contrast microscopy at a final magnification of 200× or 400× in a Makler counting chamber. A drop of 10 or 20 µL of diluted semen was introduced into the Makler chamber and covered with a coverslip. The patient was considered oligospermic if the sperm count was below 20 million/mL.
Mobility was evaluated using a simple grading system. At least five microscopic fields were assessed systematically to classify 200 SPZ. The motility of each SPZ was graded according to whether it showed rapid progressive motility (denoted as a); slow or sluggish progressive motility (b); non progressive motility (c), or immobility (d). The percentage of a+b was calculated, and asthenozoospermia was diagnosed if this percentage was less than 50%.
DNA fragmentation was assessed by SCD test [13]. In the absence of massive sperm DNA breakage and following acid denaturation and removal of nuclear proteins, dispersed DNA loops produce a characteristic halo. Sperm with fragmented DNA does not develop such a halo, or it is small. A Halosperm® kit (Halotech DNA SL, Madrid, Spain) was used following these steps: Semen was diluted in culture medium to obtain a maximum concentration of 20 million SPZ per milliliter. Agarose (100 µL) was fluidized for 5 minutes in water at 95℃ to -100℃; then the temperature was equilibrated at 37℃ for 5 minutes. Fifty microliters of prepared sperm were added to agarose and gently mixed at 37℃. A drop of 8 µL of this cell suspension was then deposited on the treated side of a slide and covered with a glass coverslip. The slide was placed on a cold surface and put in the fridge at 4℃ for 5 minutes. After removing the coverslip, it was immersed in the acid denaturing solution for 7 minutes, then incubated in a lysis solution for 25 minutes. Next, the slide was rinsed in abundant distilled water for 5 minutes and fixed with 70% ethanol, then with 100% ethanol, each time for 2 minutes. After drying, the slide was stained by the Diff-Quik reagent. The visualization was done under a bright field light microscope (MOTIC B1 Series) with ×20 and ×40 objectives. We counted 300 to 500 SPZ while identifying those with DNA fragmentation according to manufacturer instructions [14] and then calculated the DNA fragmentation index (DFI) as:
We chose to fix the DFI threshold at 18% to distinguish between two groups of patients: a high fragmentation group (HFG: DFI>18%) and a low fragmentation group (LFG: DFI ≤18%). This threshold was used by other authors, who have indicated that SDF levels above 18%, as measured by SCD, are not compatible with the initiation and maintenance of a term pregnancy [15]. The mean age of patients in HFG and LFG were 37.84±1.13 years and 36.57±1.17 years, respectively, without a significant difference.
GraphPad Prism ver. 6.00 for Windows (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. To assess the relationships between DNA fragmentation and semen parameters, we calculated the Spearman's correlation coefficient. The Mann-Whitney test was used to compare quantitative parameters between HFG and LFG. Fisher's exact test was used to detect the difference in the morphologically normal SPZ frequency between the two study groups. The data are presented as mean±standard error in a 95% confidence interval (95% CI), and statistical significance was set at 0.05. All reported p-values are from two-sided tests.
The mean sperm concentration was 15.46±15.02× 106/mL (median, 4.5×106/mL) and was distributed as follows: <5×106/mL in 50%; between 5 and 20×106/mL in 27%; and ≥20×106/mL in 23% of the men. The mean sperm motility was 46.14%±3.27%, and 27% of the men had a sperm progressive motility ≥50%. We used the cutoff of 30% (David's classification) to assess the normality of sperm morphology. In our series, 50% of the patients had 30% or more SPZ with normal morphology in their semen. Finally, the mean semen volume in all patients was 2.85±0.26 mL.
The mean DFI in our sample was 25.55%±2.49%. Among the 26 men, 19 (i.e., 73.07%) had a DFI>18%; they represent the HFG. In this group, the mean DFI was 31.22%±2.13%, while it was 10.14%±2.28% in the LFG (DFI ≤18%).
Plotting DFI with the percentage of normal SPZ in each sample did not illustrate any correlation between the two parameters (r=-0.18; p=0.68) (Table 1, Fig. 1C). According to this, there is also no significant difference in typical SPZ forms between LFG and HFG (29.50±4.50 vs. 38.14±7.00, respectively; p=0.78; Table 2). It is worth noting that 63% of patients in HFG had at least 30% morphologically normal SPZ. Even if this proportion was inferior to that of LFG (i.e., 85.71%), belonging to HFG or LFG did not correlate with the frequency of morphologically normal SPZ, distinguished as either ≥30% or <30% (odds ratio=2.77, p=0.62, 95% CI [0.27~28.41]).
Our study helped elucidate the relationship between SDF and standard semen parameters in a sample of infertile men from Eastern Algeria. We used an SCD test because it shows, like the terminal uridine nick-end labeling (TUNEL) test, a strong relationship with the sperm chromatin structure assay (SCSA) for SDF, both in infertile men and donors of known fertility [16]. We noted that the mean sperm concentration and sperm motility in our sample were beneath WHO limits, demonstrating why ICSI was the appropriate treatment in such cases.
We found a negative correlation between the DFI and both sperm motility and concentration, whereas no correlation was observed with sperm morphology, and SDF does not seem to affect sperm morphology. Conflicting data exist in the literature concerning this issue. While some authors found the same results for motility and sperm concentration, either with a SCD test [15], TUNEL assay [17], or SCSA [18], other authors did not find this correlation for either of the standard parameters [19] or only for sperm concentration [20]. The same observations have been made with regard to sperm morphology and SDF correlation [15,20].
These discrepancies could be explained either by differences in the SDF assays that have been used or by heterogeneity in the proportion of apoptotic bodies in the sperm samples used. These are factors that vary in size and density, occurring with great prevalence in men with poor quality semen [21] and explaining why some authors found, in the same sperm sample, two types of SDF, one dependent and the other independent of semen quality [22]. Sperm selection by swim-up and/or migration in a discontinuous density gradient should affect SDF determination by eliminating apoptotic bodies and highly fragmented sperm [22]. Such selection could explain the discrepancies between the studies. It could also explain the fact that we did not find a significant difference in motility between LFG and HFG, since this parameter could be ameliorated by DGC. Additionally, the most frequent abnormality we found in the LFG (92%) was asthenospermia, and this could contribute to the explanation of the non-significant difference in motility between LFG and HFG.
We used 18% DFI as the threshold value to distinguish between LFG and HFG. To make this choice, we were inspired from other publications using the same DNA fragmentation kit as ours [15]. Threshold values for infertility depend on the SDF assay type: Studies using an SCD assay use a threshold varying from 17% to 22.75% [15,23,24], whereas those using SCSA [25] or a TUNEL assay [24,26] set the cut-off at 27% to 30% or 12% to 20%, respectively. We should note that, in our study, all patients with standard semen parameter abnormalities had a DFI>18%, and that 97.73% of men in HFG, but only 30% in LFG, suffer from at least one standard sperm parameter abnormality. This could demonstrate indirectly that impairment of semen parameters is associated with an increase in SDF. Additionally, it is well known that the sperm DNA damage factor can be either testicular or extra testicular; thus if SDF is due, for example, to a failure in DNA break repair, other aspects of spermatogenesis can be affected, resulting in sperm number, motility, or morphology abnormalities. It can also be speculated that, if SDF is due to reactive oxygen species (ROS), then a relationship to sperm motility could be expected because ROS affect lipid peroxidation of sperm membranes rich with unsaturated fatty acids [27]. Furthermore, in highly differentiated elongated spermatids or mature spermatozoa, apoptotic events may be modified [28], so that SPZ mitochondrial activity, motility, and morphology can be normal although DNA is fragmented. Indeed, SPZ displaying translocation of membrane phosphatidylserine, as diagnosed by annexin V positive staining, were found in sperm fractions with both high and low motility [29], as well as in morphologically normal SPZ [30].
Finally, we did not find any correlation between semen volume and DFI, and this could be explained by the fact that semen is mainly composed of seminal fluid secretion from annex glands, which makes volume independent of SDF.
Our results show that the semen parameters of sperm concentration and motility were inversely correlated with SDF, whereas no correlation was observed between SDF and the other parameters. Taken together with the results of other authors who have even claimed that sperm DNA integrity measurement is more reproducible and more objective than conventional parameters [31,32], our results allow us to propose that SDF screening should be used as a complementary sperm parameter participating in semen quality evaluation by providing useful information in the diagnosis of male infertility.
Figures and Tables
Fig. 1
DNA fragmentation index (DFI) correlation with standard semen parameters. Linear regression of sperm concentration (r=-0.65, p=0.0003) (A), motility (r=-0.45, p=0.03) (B), and normal morphology (r=-0.18, p=0.68) (C), on DFI. SPZ: spermatozoon.

Table 1
Correlation of DNA fragmentation index with standard semen parameters

| Variable | ra | 95% CI |
|---|---|---|
| Sperm volume | 0.24 | [-0.17~0.58] |
| Sperm concentration | -0.65** | [-0.84~-0.38] |
| Sperm motility | -0.45* | [-0.73~-0.02] |
| Percentage of morphologically normal SPZ | -0.18 | [-0.66~0.39] |
ACKNOWLEDGEMENTS
The authors thank Dr Benbouhadja S., the director of Ibn Rochd clinic; Dr. Zoghmar A., the chief of the medically assisted procreation laboratory; and the entire laboratory staff at this clinic.
References
1. World Health Organization (WHO). WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO;2010.
2. Gao J, Gao ES, Walker M, Yang Q, Wu JQ, Zhu QX, et al. Reference values of semen parameters for healthy Chinese men. Urol Int. 2008; 81:256–262.

3. Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006; 85:629–634.

4. Barratt CL, Björndahl L, Menkveld R, Mortimer D. ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance. Hum Reprod. 2011; 26:3207–3212.

5. Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001; 345:1388–1393.

6. Lewis SE. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007; 134:31–40.

7. Barratt CL, Aitken RJ, Björndahl L, Carrell DT, de Boer P, Kvist U, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications: a position report. Hum Reprod. 2010; 25:824–838.
8. Nijs M, Creemers E, Cox A, Franssen K, Janssen M, Vanheusden E, et al. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod Biomed Online. 2009; 19:671–684.

9. Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, et al. Study For Future Families Research Group. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003; 111:414–420.

10. Iwamoto T, Nozawa S, Yoshiike M, Namiki M, Koh E, Kanaya J, et al. Semen quality of fertile Japanese men: a cross-sectional population-based study of 792 men. BMJ Open. 2013; 3:doi: 10.1136/bmjopen-2012-002223.

11. World Health Organization (WHO). WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press;1999.
12. David G. Editorial: sperm banks in France. Arch Fr Pediatr. 1975; 32:401–404.
13. Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003; 24:59–66.
14. Halotech DNA [Internet]. Madrid: Halotech DNA;accessed 2014 Dec 10. Available from: http://www.halotechdna.com/productlist/.
15. Velez de la Calle JF, Muller A, Walschaerts M, Clavere JL, Jimenez C, Wittemer C, et al. Sperm deoxyribonucleic acid fragmentation as assessed by the sperm chromatin dispersion test in assisted reproductive technology programs: results of a large prospective multicenter study. Fertil Steril. 2008; 90:1792–1799.

16. Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006; 27:53–59.

17. Brahem S, Mehdi M, Landolsi H, Mougou S, Elghezal H, Saad A. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology. 2011; 78:792–796.

18. Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian J Androl. 2008; 10:786–790.

19. Khalili MA, Aghaie-Maybodi F, Anvari M, Talebi AR. Sperm nuclear DNA in ejaculates of fertile and infertile men: correlation with semen parameters. Urol J. 2006; 3:154–159.
20. Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009; 91:1801–1805.

21. Marchiani S, Tamburrino L, Forti G, Baldi E, Muratori M. M540 bodies and their impact on flow cytometric analyses of human spermatozoa. Soc Reprod Fertil Suppl. 2007; 65:509–514.
22. Muratori M, Marchiani S, Tamburrino L, Tocci V, Failli P, Forti G, et al. Nuclear staining identifies two populations of human sperm with different DNA fragmentation extent and relationship with semen parameters. Hum Reprod. 2008; 23:1035–1043.

23. Nuñez-Calonge R, Caballero P, López-Fernández C, Guijarro JA, Fernández JL, Johnston S, et al. An improved experimental model for understanding the impact of sperm DNA fragmentation on human pregnancy following ICSI. Reprod Sci. 2012; 19:1163–1168.

24. Ribas-Maynou J, García-Peiró A, Fernández-Encinas A, Abad C, Amengual MJ, Prada E, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology. 2013; 1:715–722.

25. Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004; 19:1401–1408.

26. Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002; 17:3122–3128.

27. Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006; 8:11–29.

28. Hikim AP, Wang C, Leung A, Swerdloff RS. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1995; 136:2770–2775.

29. Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000; 15:1338–1344.

30. Høst E, Lindenberg S, Kahn JA, Christensen F. DNA strand breaks in human sperm cells: a comparison between men with normal and oligozoospermic sperm samples. Acta Obstet Gynecol Scand. 1999; 78:336–339.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


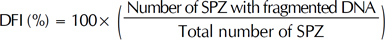

 XML Download
XML Download