Abstract
Purpose
In epidemiological studies, there are various associations of androgen receptor (AR) CAG with several diseases or phenotypes. However, the relationship between CAG repeat length and metabolic syndrome (MS) remains unclear, especially in Asian populations. This study was designed to evaluate the relationship between AR CAG repeat length polymorphism and MS in a Korean male population.
Materials and Methods
We explored the relationship between AR CAG repeat length polymorphism and MS in a Korean male population (n=337) from 2013 to 2014. AR CAG repeat were determined by microsatellite fragment sizing. Components of MS and laboratory data (lipid profile, fasting glucose, and glycated hemoglobin (HbA1c)) were analyzed with AR CAG repeat length.
Results
The mean AR CAG repeat length was 22.3±4.7. Sixty-nine men (20.5%) were diagnosed with MS. Men with MS showed significantly longer AR CAG repeat lengths compared with men without MS (26.2 vs. 21.4, p<0.001). With increasing CAG repeat, the number of components meeting the NCEP criteria increased significantly. AR CAG repeat length was associated significantly with high density lipoprotein (HDL), triglyceride, and HbA1c levels. In the multivariate analysis, CAG repeat length, waist circumference, and levels of HDL were independently associated with MS. (odds ratio (OR)=1.37, 1.19 and 0.90, p<0.001, 0.045, and 0.001, respectively).
The effects of androgen are mediated by activation of the androgen receptor (AR). The X-chromosomal AR gene is located on Xq11-12 and comprises eight exons. It contains a highly polymorphic region in exon 1 with a variable number of CAG repeats, (CAG)n, which encodes a polyglutamine tract in the N-terminal transactivation domain of the AR [1234].
The trinucleotide (CAG)n repeat polymorphism is thought to regulate AR activity, with longer alleles conferring reduced receptor activity [56]. Thus, it is commonly supposed that there is a negative association between CAG repeat length and AR activity.
The length of CAG repeats is about 10 to 30 iterations, and in the Caucasian population it is repeated 22 times on average [7]. However, there are significant ethnic variations in allele distribution of the AR (CAG)n repeats [2345]. Unfortunately, there are few studies on CAG repeat length in Asian male populations.
Metabolic syndrome (MS) is a combination of metabolic disturbances. The core components of MS include obesity, insulin resistance, dyslipidemia, and hypertension [8]. In recent studies, testosterone level seems to be associated with the components of MS and MS itself [910]. However, there are several limitations to measuring testosterone precisely, and sometimes testosterone does not correlate with clinical symptoms. Most androgen related effects are mediated thorough the AR; therefore, MS has been hypothesized to be associated with CAG repeat length polymorphisms rather than testosterone [9].
However, the relationship between CAG repeat and MS remains unclear and research is insufficient in Asian populations. In this study, we investigated the relationship between CAG repeats and MS in a Korean male population.
A cross-sectional study was carried out at the Korea University Guro Hospital, Seoul, Korea from June 2013 to May 2014. The study protocol was reviewed and approved by the institutional review board of Korea University Gur Hospital. We recruited Korean men over the age of 40 years. Many of the subjects were recruited from a benign prostate hyperplasia/lower urinary tract symptoms screening program. At the time of recruitment, informed consent was obtained and the details of patients' characteristics, medical histories, and drug histories were recorded.
Clinical and biochemical assessments of the patients were made using a questionnaire and by laboratory tests. Exclusion criteria included the presence of Klinefelter syndrome, Kallmann syndrome, primary hypogonadism, hypogonadotropic hypogonadism, and previous medical use of testosterone or androgen products.
To minimize the alteration of circadian variations, blood samples were collected between 8 AM and 11 AM from all patients. Concentrations of total testosterone and free testosterone were measured using radioimmunoassay kits (Testo-RIA-CT; DIAsource ImmunoAssays, Nivelles, Belgium and Coat-A-Count Free Testosterone; Diagnostic Products Corporation, Los Angeles, CA, USA, respectively). Height and weight were checked and the body mass index (BMI) is calculated as weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference was measured with a tape measure at the level midway between the lowest rib margin and the iliac crest.
MS was diagnosed according to the National Cholesterol Education Program-Adult Treatment Panel III criteria. It requires the presence of 3 or more of the following components: central obesity (waist circumference>90 cm), high density lipoprotein (HDL) cholesterol<40 mg/dL, triglycerides≥150 mg/dL, fasting glucose≥100 mg/dL, or blood pressure≥130/85 mmHg (or taking antihypertensive drug treatment) [11].
Microsatellite fragment sizing was performed to measure CAG repeats, according to the method of Stanworth et al [1213]. Genomic DNA was extracted from peripheral blood and polymerase chain reaction (PCR) was performed to amplify the region of the AR gene that included CAG. The DNA was stored at −80℃ until analysis. DNA was amplified by PCR using PCR master mix (ABGene, Epsom, UK). Amplification was carried out using an automatic thermal cycler applying the following PCR conditions: 94℃ for 5 minutes; followed by 32 cycles of 94℃ for 1 minute, 58℃ for 1 minute and 72℃ for 1 minute; followed by final extension at 72℃ for 7 minutes and denaturation at 96℃ for 5 minutes. After magnetic separation of the PCR products, each sample was analyzed using a capillary-based AB 3730 automated sequencer (Applied Biosystems, Warrington, UK) which produces an electropherogram from which the DNA sequence can be derived [1213].
Data are expressed as the mean±standard deviation or number (percentage). The p-values<0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS software ver. 20.0 (IBM Co., Armonk, NY, USA).
Pearson's correlation coefficients (r) were calculated to study the associations among clinical factors (MS, age, height, weight, BMI, waist circumference, fasting blood sugar, total cholesterol, HDL, low density lipoprotein [LDL], triglyceride, glycated hemoglobin [HbA1c]). Univariate and multivariate regression analyses were completed to assess the independent associations among the factors.
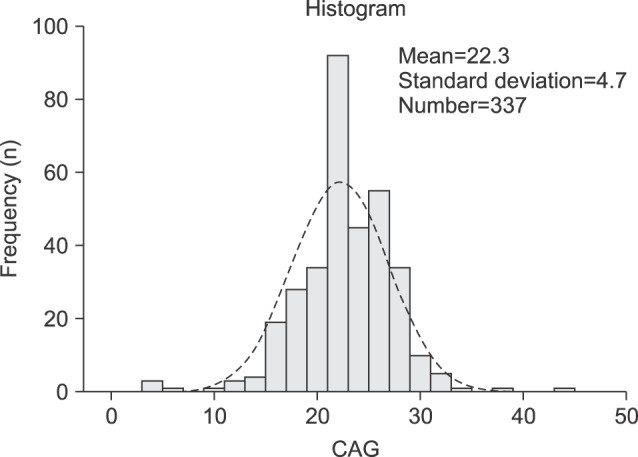
A total of 376 Korean men were recruited, 21 men withdrew consent, and 18 men were excluded by exclusion criteria (previous testosterone use). Therefore, 337 Korean men were enrolled in the study. The mean age of the subjects was 58.7±11.0 years (range, 40∼90 years). The mean BMI was 24.3±2.7 kg/m2 (range, 17.1∼35.1 kg/m2) (Table 1). The mean AR CAG repeat length was 22.3±4.7 (Fig. 1). Sixty-nine men (20.5%) were diagnosed with MS. A comparison of the demographic characteristics and laboratory data between the men with MS and those without MS showed significant differences in age, CAG repeat length, weight, BMI, waist circumference, and levels of HDL, triglyceride, fasting glucose, and HbA1c.
Men with MS showed significantly longer AR CAG repeat lengths than men without MS (26.2 vs. 21.4, p<0.001; Table 2). The number of MS criteria increased meaningfully as CAG repeat length increased (R2=0.119, p=0.001; Fig. 2).
In the correlation analysis between CAG and components of MS, AR CAG repeat length was not associated with fasting glucose, LDL, or total cholesterol levels (p=0.224, 0.496, and 0.339, respectively). However, CAG repeat revealed a significant association with HDL (r=−0.244, p<0.001), triglyceride (r=0.276, p<0.001), and HbA1c (r=0.201, p<0.001) levels (Fig. 3).
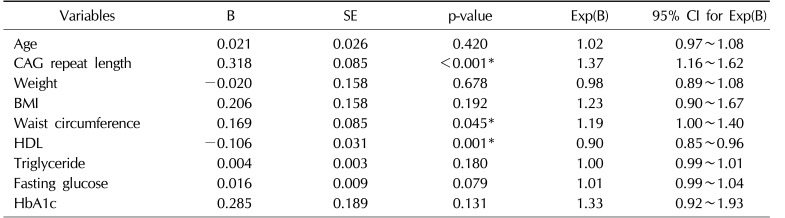
Univariate analysis revealed that MS was significantly influenced by age, CAG repeat length, weight, BMI, waist circumference, levels of HDL, triglyceride, fasting glucose, and HbA1c. In the multivariate analysis of these factors, CAG repeat length, waist circumference, and levels of HDL were independently associated with MS (odds ratio=1.37, 1.19, and 0.90; p<0.001, 0.045, and 0.001, respectively) (Table 3).
Androgens mediate their effects primarily through activation of the AR [14]. The N-terminal domain, which contains the transactivating capacity, contains a glutamine repeat, commonly referred to as the CAG repeat polymorphism [9]. The length of the CAG repeat is thought to regulate AR activity; longer CAG repeat lengths lead to lower AR activity.
The mean number of CAG repeats in the present study was 22.3±4.7, which is slightly higher than that in Caucasians [15]. The only previous study on AR CAG repeat length in Korean men determined the mean number repeats to be 23; however, that study assessed only 42 hypogonadal men [16]. Therefore, our study is meaningful in that it presents the mean number of AR CAG repeats of a larger group of Korean male patients. More studies are needed to determine the mean AR CAG repeat length of Korean men.
MS includes a cluster of metabolic disturbances, specifically, obesity, hypertension, diabetes, low HDL cholesterol, and hypertriglyceridemia [810]. Recently, the relationship between MS and testosterone deficiency syndrome has become a research focus [1718]. Low testosterone levels have been associated with the components of MS and MS itself [910].
Therefore, AR polymorphisms could adjust the association between MS and testosterone. Indeed, several recent studies presented data on the relevance between CAG repeats and the components of MS [41920]. However, previous reports on the association of AR gene polymorphism with MS are contradictory [9].
Our study showed an association between CAG repeat length and MS. In addition, as the CAG repeat length increased, the number of the components of MS increased significantly. Furthermore, AR CAG repeat length showed a significant association with HbA1c levels. This was similar to the results of Zitzmann et al [4], who demonstrated a positive independent correlation of the CAG repeat number with body fat content, leptin, and insulin. They suggested the possible mechanism that longer CAG repeat length caused MS. CAG repeat length showed a modulatory influence on body fat and a low CAG repeat number is related to low body fat mass. The number of CAG also showed a positive relationship with the level of insulin [4]. These results propose that a lower number of CAG repeats is helpful to metabolic parameters [4]. Similarly, Stanworth et al [13] showed a negative association of testosterone, and a positive association of CAG with obesity and leptin. The associations of AR CAG with leptin and obesity were independent of levels of testosterone, estradiol, gonadotropins, and age [13]. However, Skjaerpe et al [9] reported conflicting results: an inverse association between CAG repeat length and the number of components of MS in elderly men. Skjaerpe's study [9] had a limited number of subjects (172 patients), and most of them were elderly patients. There was a difference in the CAG repeat length according to the number of components of MS; however, there was no difference according to the presence of MS. Thus, it is difficult to generalize from the results of their study.
Our study had several limitations. First, the study had a small sample size and was performed in a single center; therefore, our results need to be confirmed in larger studies. Second, although the age of the subjects varied widely, they might not have represented the general population.
Despite these limitations, our study is meaningful because it demonstrated associations between CAG repeats and MS. In the multivariate analysis, a longer CAG repeat length was an independent risk factor for MS. To the best of our knowledge, this is the first time that such an association between CAG repeats and MS has been identified in an Asian population. Further prospective studies with larger sample sizes and involving other centers with controlled environmental variables are warranted.
CAG repeat length was related to MS and laboratory test results, such as those for HDL, TG, and HbA1c in a Korean male population. Longer CAG repeat length was recognized as an independent risk factor for MS in Korean males. Further studies with larger sample sizes are required to determine the clinical significance of the correlation between AR CAG repeat length and MS in Korean males.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (R1304182).
Notes
Author Contribution: Conceived the study and drafted the manuscript: Kim Jong Wook. Collected the data, performed the statistical analysis, and helped to draft the manuscript: Bae YD, Ahn ST. Helped to conceive the study and drafted the manuscript: Kim Jin Wook, Kim JJ. Conceived the study and participated in its design and coordination: Moon DG.
References
1. Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988; 240:327–330. PMID: 3353727.

2. Goutou M, Sakka C, Stakias N, Stefanidis I, Koukoulis GN. AR CAG repeat length is not associated with serum gonadal steroids and lipid levels in healthy men. Int J Androl. 2009; 32:616–622. PMID: 18657194.

3. Travison TG, Shackelton R, Araujo AB, Morley JE, Williams RE, Clark RV, et al. Frailty, serum androgens, and the CAG repeat polymorphism: results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2010; 95:2746–2754. PMID: 20410235.

4. Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003; 46:31–39. PMID: 12637980.

5. Ackerman CM, Lowe LP, Lee H, Hayes MG, Dyer AR, Metzger BE, et al. Ethnic variation in allele distribution of the androgen receptor (AR) (CAG)n repeat. J Androl. 2012; 33:210–215. PMID: 21597087.

6. Mhatre AN, Trifiro MA, Kaufman M, Kazemi-Esfarjani P, Figlewicz D, Rouleau G, et al. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1993; 5:184–188. PMID: 8252045.

7. Lundin KB, Giwercman A, Richthoff J, Abrahamsson PA, Giwercman YL. No association between mutations in the human androgen receptor GGN repeat and inter-sex conditions. Mol Hum Reprod. 2003; 9:375–379. PMID: 12802043.

8. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23:469–480. PMID: 16681555.
9. Skjaerpe PA, Giwercman YL, Giwercman A, Svartberg J. Androgen receptor gene polymorphism and the metabolic syndrome in 60–80 years old Norwegian men. Int J Androl. 2010; 33:500–506. PMID: 19207614.
10. Svartberg J. Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res. 2007; 19:124–128. PMID: 16858366.

11. Lauer MS, Fontanarosa PB. Updated guidelines for cholesterol management. JAMA. 2001; 285:2508–2509. PMID: 11368705.

12. Stanworth RD, Akhtar S, Channer KS, Jones TH. The role of androgen receptor CAG repeat polymorphism and other factors which affect the clinical response to testosterone replacement in metabolic syndrome and type 2 diabetes: TIMES2 sub-study. Eur J Endocrinol. 2013; 170:193–200. PMID: 24165020.

13. Stanworth RD, Kapoor D, Channer KS, Jones TH. Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur J Endocrinol. 2008; 159:739–746. PMID: 18805913.

14. Mouritsen A, Hagen CP, Sørensen K, Aksglaede L, Mieritz MG, Main KM, et al. Androgen receptor CAG repeat length is associated with body fat and serum SHBG in boys: a prospective cohort study. J Clin Endocrinol Metab. 2013; 98:E605–E609. PMID: 23393169.

15. Zitzmann M, Nieschlag E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl. 2003; 26:76–83. PMID: 12641825.
16. Kim MJ, Kim JT, Cho SW, Kim SW, Shin CS, Park KS, et al. Androgen receptor gene CAG repeat polymorphism and effect of testosterone therapy in hypogonadal men in Korea. Endocrinol Metab. 2011; 26:225–231.

17. Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007; 14:226–234. PMID: 17940444.

18. Kapoor D, Jones TH. Androgen deficiency as a predictor of metabolic syndrome in aging men: an opportunity for intervention? Drugs Aging. 2008; 25:357–369. PMID: 18447401.
19. Hersberger M, Muntwyler J, Funke H, Marti-Jaun J, Schulte H, Assmann G, et al. The CAG repeat polymorphism in the androgen receptor gene is associated with HDL-cholesterol but not with coronary atherosclerosis or myocardial infarction. Clin Chem. 2005; 51:1110–1115. PMID: 15890890.

20. Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, et al. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol (1985). 2005; 98:132–137. PMID: 15377647.

Fig. 2
Association and correlation analysis between androgen receptor CAG repeat length and metabolic syndrome. As the CAG repeat length increased, the number of components from the National Cholesterol Education Program-Adult Treatment Panel (NCEP) criteria increased significantly (R2=0.119, p=0.001).
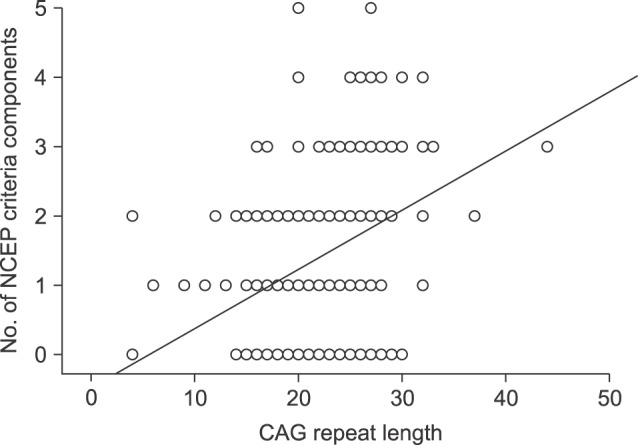
Fig. 3
Androgen receptor CAG repeat length showed a significant association with high density lipoprotein (HDL) (r=−0.244, p<0.001), triglyceride (r=0.276, p<0.001), and glycated hemoglobin (HbA1c) (r=0.201, p<0.001) levels.
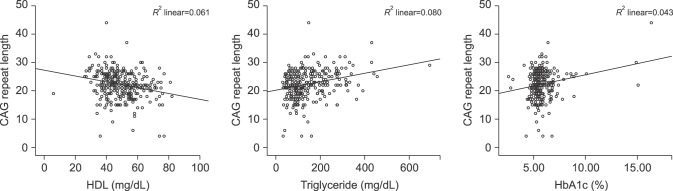
Table 1
Baseline characteristics of the study subjects
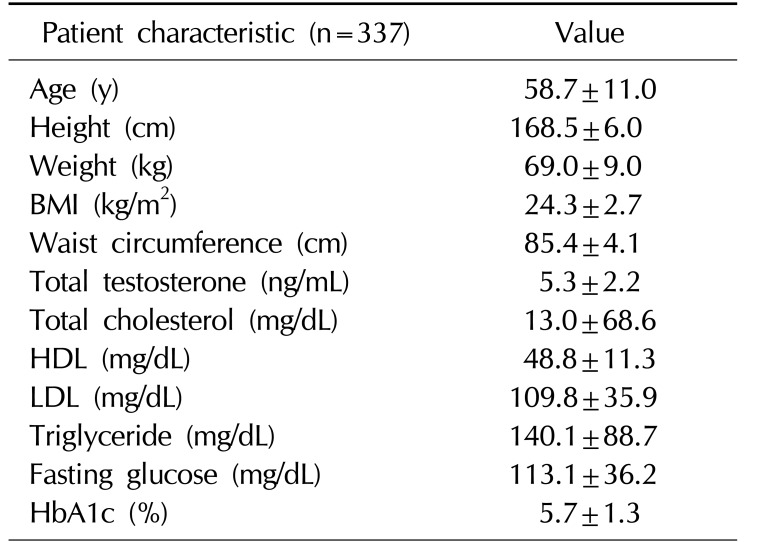
Table 2
Comparison of demographic characteristics and laboratory data between men with MS and without MS
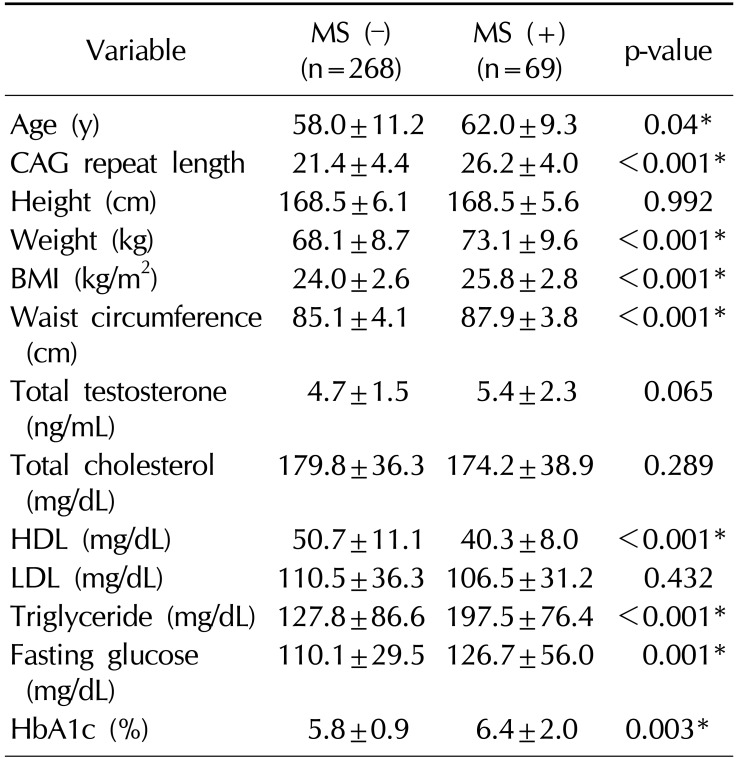
Table 3
Multivariate analysis associated with prevalence of metabolic syndrome




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download