Abstract
As the age of paternity rises in the developed world, issues of chronic disease may affect prospective fathers. Given the high prevalence of hypertension, researchers have begun to explore the relationship between hypertensive disease and male fertility. The current literature suggests an association between hypertension and semen quality. The use of various antihypertensive medications has also been linked to impaired semen parameters, making it difficult to discern whether the association exists with hypertension or its treatment. Further investigation is warranted to determine whether the observed associations are causal.
The average age of paternity is rising in America. Data from the Centers for Disease Control have shown that the birth rate for fathers from ages 25 to 29 years decreased 15%, while the birthrate for fathers from ages 35 to 39 years increased by 18% from 2000 to 2013 [1]. Importantly, as men age, they are more prone to develop chronic illnesses. Considering the link between several medical diseases and impaired semen quality [2], it is important to investigate the potential impact of chronic illness on male fertility.
Hypertension is the most common chronic illness reported among men in the USA, affecting 30% of adult males [3]. Not surprisingly, the use of prescription antihypertensive medications is also common. Antihypertensive medications lead all other categories in annual prescriptions, with 705 million prescriptions dispensed in 2014 [4]. Despite this, the relationship between hypertension and male fertility has received limited attention.
The prior literature has posited a link between the infertility and the metabolic syndrome, a cluster of conditions including insulin insensitivity, obesity, hyperlipidemia, and hypertension. For example, several groups have highlighted a collection of studies that suggested an association between infertility and obesity/high body mass index, diabetes, and dyslipidemia [5678]. Ventimiglia et al [9] explored the association between medical comorbidity and semen quality, reporting an inverse relationship and implying that metabolic syndrome had a negative impact on reproductive health. Nevertheless, the literature regarding hypertension in isolation, or its treatment, is limited.
Rare studies have linked hypertension to some aspects of sperm physiology. A Brazilian group employed a rat model for renovascular hypertension to demonstrate decreased sexual behavior and impaired spermatogenesis, which they attributed to imbalances in prolactin, testosterone and follicle stimulating hormone [10]. In addition, an Italian group found higher levels of clusterin, a glycoprotein associated with abnormal sperm morphology, in a small cohort hypertensive men compared to normotensive men [11]. These studies were small in scale and exploratory in nature.
We utilized the Stanford Infertility data to investigate the association between hypertension and semen quality. The study cohort consists of men who were evaluated for infertility as part of an infertile couple between 1994 and 2011 at the Stanford Reproductive Endocrinology and Infertility Center. This center evaluates and treats infertile couples with both male and female infertility. The laboratory performs a high volume of semen analyses for fertility evaluation and sperm preparations for use with assisted reproductive techniques. Men evaluated for infertility were self-referred, or were referred by an internist, gynecologist, urologist, or reproductive endocrinologist. The methods used for analysis of semen (sperm concentration, motility, volume, morphology) have been previously described [121314].
After receiving approval from the Institutional Review Board, the assembled cohort was linked to insurance claims and electronic medical record data for each patient using unique medical record numbers. We collected data including medical diagnoses (International Classification of Disease, 9th edition, ICD-9), procedures (Current Procedural Terminology, CPT), and medications prescribed. Complete medication data were available beginning in 2008. For antihypertensive medications, we classified them into five categories: beta beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers, and diuretics.
Men with an ICD-9 code between 401.0 and 405.9 were considered to have a diagnosis of hypertension. To capture prevalent disease, only men who were diagnosed with hypertension prior to or within one year after semen analysis were included. Abnormal semen parameters were defined based on the World Health Organization (WHO) 5th edition of the Manual on Semen Analyses (i.e., subfertile values: semen volume <1.5 mL, sperm concentration <15 mol/mL, sperm motility <40%, sperm morphology <14% normal forms) [14]. Antihypertensive medications were only included if taken in the year prior to the semen analysis.
To compare men with hypertension and those without, we used the Wilcoxon rank-sum test for age, and chi-square for age group and year of evaluation. Semen tests were analyzed in a mixed effect linear model with repeated measures. All semen tests were square-root-transformed per convention for the analyses given their non-Gaussian distribution. The percentage of men with Suboptimal Semen Parameters according to WHO 5th edition criteria were analyzed using a generalized linear mixed model with repeated measures. The tests were adjusted for patient age and the year of fertility evaluation. For the comparisons between men who took diuretics and those who did not, the Wilcoxon rank-sum test was also applied due to their limited number. All p-values were two-sided, with p<0.05 considered statistically significant. Analyses were performed using SAS ver. 9.3 (SAS Institute, Cary, NC, USA).
Men with hypertension were found to be more likely to have one or more semen abnormalities compared to normotensive men [2]. Moreover, compared to men without hypertension, men diagnosed with hypertension demonstrated impaired semen parameters [15]. Hypertensive men had a lower semen volume (2.1 mL vs. 3.0 mL, p<0.001), sperm motility (41.0% vs. 47.0%, p=0.008), total sperm count (103.8 vs. 147.0, p=0.005) and total motile sperm count (43.1 vs. 65.9, p=0.003). When stratifying men by WHO (5th edition) semen quality criteria, a higher prevalence of men with hypertension had subfertile semen volume (18.1% vs. 10.0%, p=0.03), sperm concentration (19.4% vs. 12.2%, p=0.02), and total motile sperm count (25.8% vs. 15.5%, p=0.01). There were also trends toward suboptimal motility and total motile count in men with hypertension, but these were not statistically significant (Table 1).
We next looked at men for whom medication data were available (n=1,167). Of those men, 88 men were taking 1 antihypertensive medication and 45 were taking 2 or more antihypertensive medications. Compared to men not taking medications, men taking 1 antihypertensive medication had a statistically significant decrease in semen volume (2.0 mL vs. 2.5 mL, p=0.05) with a trend toward a lower sperm count (p=0.07).
We then stratified men by individual class of antihypertensive medication [15]. Several differences were identified between classes of antihypertensives. Men who were taking beta-blockers were noted to have decreased volume, concentration and motility compared to men not taking medications (p<0.05). Men taking calcium channel blockers had relatively decreased sperm concentration. Men taking angiotensin-receptor blockers had relatively increased volume and decreased sperm concentration. Men taking ACEIs had relatively decreased volume and decreased motility. Lastly, men taking diuretics had relatively decreased volume (Table 2).
Infertility may be a harbinger of health. As such, a man's reproductive fitness may also reflect his somatic fitness. Studies in the USA and Europe have demonstrated higher mortality rates among infertile men [1617]. In addition, higher rates of certain types of cancers have also been reported among infertile men in the years following a fertility evaluation [18192021]. Recently, data from a large USA insurance claims database demonstrated a higher incidence of heart disease and diabetes among infertile men [22]. Importantly, a higher incidence of hypertension was not identified in this group, suggesting that male infertility is not a risk factor for hypertension itself.
Existing data suggest an association between hypertension and impaired semen quality. Men diagnosed with hypertension have a lower semen volume, sperm motility, total sperm count, and motile sperm count relative to men in the cohort who did not carry a diagnosis of hypertension. Importantly, more men with a diagnosis of hypertension had impaired semen volume, concentration, and total motile count, according to WHO 5th edition criteria for subfertile semen parameters. Moreover, the use of beta-blockers was associated with lower semen volume, concentration, motility, total sperm count, and total motile sperm count, while men taking other antihypertensives had more isolated impairments in semen parameters.
The direct end-organ effects of hypertension on the arteries and kidneys have been studied in depth, but the effect on the testes is not well characterized. Prior research has addressed the relationship between hypertension and the endocrine axis, which may affect reproductive ability. For example, in a cross-sectional study of 1,548 men, Svartberg et al [23] demonstrated an inverse association between total testosterone level and systolic blood pressure. In a case-control study of 110 newly diagnosed hypertensive men, Fogari et al [24] showed a 10% reduction in total testosterone levels compared to normotensive men. However, given study design, causal pathways between hypertension and testicular function cannot be inferred.
While the etiology of the association between hypertension and semen quality remains unknown, the relationship between somatic health and semen production has been reported [92425]. Authors have suggested several plausible hypotheses. For example, the fetal origins of disease theory posits a common in utero exposure could lead to both infertility and hypertension [262728]. In addition, the coexistence with other components of the metabolic syndrome (e.g., obesity, hyperlipidemia) have been demonstrated to associate with impaired semen quality [78].
The etiology may also relate to treatment rather than the disease alone. The current report identified multiple abnormalities associated with beta-blockers but not other individual classes of antihypertensives. This is particularly relevant given that beta-blockers represent one of the most commonly medications prescribed, with over 85.3 million prescriptions dispensed in the USA in 2014 [4].
Importantly, several medications have been investigated previously for their impact on fertility. For example, retrograde ejaculation is known to be a common side effect of alpha-blockers and consequently would decrease semen volume [29]. Thus, the non-selective alpha-blocker phenoxybenzamine was investigated as a male oral contraceptive in the 1980s [30]. In addition, a case report documented a pregnancy for an infertile couple following the discontinuation of the calcium channel-blocker nifidipine [31]. An in vitro study suggested that certain receptors for normal acrosomal reaction were reversibly impaired by calcium-channel blockers [32].
Interestingly, not all antihypertensives have been associated with impaired semen quality. Mbah et al [33] examined ACEIs through a placebo-controlled randomized study of normotensive men with idiopathic oligospermia and actually showed that low-dose lisinopril treatment resulted in improvements in the form of higher percent motility and decreased abnormal morphology, although average volume remain unchanged. In the current report, no significant improvements were seen with any class of antihypertensives while beta blockers were associated with significant impairments in semen quality.
It is important to note several limitations to the current literature examining male fertility and hypertension. First, the populations consisted of men who presented specifically for evaluation of infertility, and therefore may not be generalizable to the general population. Next, the retrospective nature of most studies leads to the possibility of bias.
Given the prevalence of hypertension and its treatment in the USA, the current literature suggests a novel observation about the possible fertility implications of a common chronic disease (and treatment), and is applicable to a growing category of older fathers. While infertile men do not have a higher risk for developing hypertension, they do have an elevated risk for vascular disease [22]. Nevertheless, further investigation of hypertension and semen quality appears warranted to understand if the reported associations are causal.
References
1. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015; 64:1–65.
2. Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril. 2015; 103:66–71. PMID: 25497466.

3. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013; (133):1–8.
4. Aitken M. Medicines use and spending shifts: a review of the use of medicines in the U.S. in 2014. Parsippany (NJ): IMS Institute for Health Informatics;2015.
5. Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 2009; 41:777–784. PMID: 19381857.

6. Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014; 29:193–200. PMID: 24306102.

7. Schisterman EF, Mumford SL, Chen Z, Browne RW, Boyd Barr D, Kim S, et al. Lipid concentrations and semen quality: the LIFE study. Andrology. 2014; 2:408–415. PMID: 24596332.

8. Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013; 19:221–231. PMID: 23242914.

9. Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril. 2015; 104:48–55. PMID: 26006735.
10. Breigeiron MK, Lucion AB, Sanvitto GL. Effects of renovascular hypertension on reproductive function in male rats. Life Sci. 2007; 80:1627–1634. PMID: 17316702.

11. Muciaccia B, Pensini S, Culasso F, Padula F, Paoli D, Gandini L, et al. Higher clusterin immunolabeling and sperm DNA damage levels in hypertensive men compared with controls. Hum Reprod. 2012; 27:2267–2276. PMID: 22647452.

12. Coetzee K, Kruger TF, Lombard CJ. Repeatability and variance analysis on multiple computer-assisted (IVOS) sperm morphology readings. Andrologia. 1999; 31:163–168. PMID: 10363121.

13. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988; 49:112–117. PMID: 3335257.

14. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010; 16:231–245. PMID: 19934213.

15. Guo D, Li S, Behr B, Eisenberg M. PD52-12 The impact of hypertension and antihypertensives on semen quality. J Urol. 2015; 193:e1117.

16. Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, et al. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014; 29:1567–1574. PMID: 24838701.

17. Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009; 170:559–565. PMID: 19635736.

18. Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015; 193:1596–1601. PMID: 25463997.

19. Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000; 321:789–792. PMID: 11009515.

20. Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009; 169:351–356. PMID: 19237718.

21. Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010; 116:2140–2147. PMID: 20309846.

22. Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril. 2016; 105:629–636. PMID: 26674559.

23. Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. 2004; 150:65–71. PMID: 14713281.
24. Fogari R, Zoppi A, Preti P, Rinaldi A, Marasi G, Vanasia A, et al. Sexual activity and plasma testosterone levels in hypertensive males. Am J Hypertens. 2002; 15:217–221. PMID: 11939610.

25. Salonia A, Matloob R, Gallina A, Abdollah F, Saccà A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009; 56:1025–1031. PMID: 19297076.

27. Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016; 96:55–97. PMID: 26582516.

28. Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001; 16:972–978. PMID: 11331648.

29. Samplaski MK, Nangia AK. Adverse effects of common medications on male fertility. Nat Rev Urol. 2015; 12:401–413. PMID: 26101108.

30. Homonnai ZT, Shilon M, Paz GF. Phenoxybenzamine: an effective male contraceptive pill. Contraception. 1984; 29:479–491. PMID: 6430643.
31. Hershlag A, Cooper GW, Benoff S. Pregnancy following discontinuation of a calcium channel blocker in the male partner. Hum Reprod. 1995; 10:599–606. PMID: 7782439.

32. Benoff S, Cooper GW, Hurley I, Mandel FS, Rosenfeld DL, Scholl GM, et al. The effect of calcium ion channel blockers on sperm fertilization potential. Fertil Steril. 1994; 62:606–617. PMID: 8062958.
33. Mbah AU, Ndukwu GO, Ghasi SI, Shu EN, Ozoemena FN, Mbah JO, et al. Low-dose lisinopril in normotensive men with idiopathic oligospermia and infertility: a 5-year randomized, controlled, crossover pilot study. Clin Pharmacol Ther. 2012; 91:582–589. PMID: 22378155.

Table 1
Comparison of men with hypertension and men without hypertension, according to absolute semen parameters and World Health Organization (WHO) 5th criteria suboptimal semen parametersa
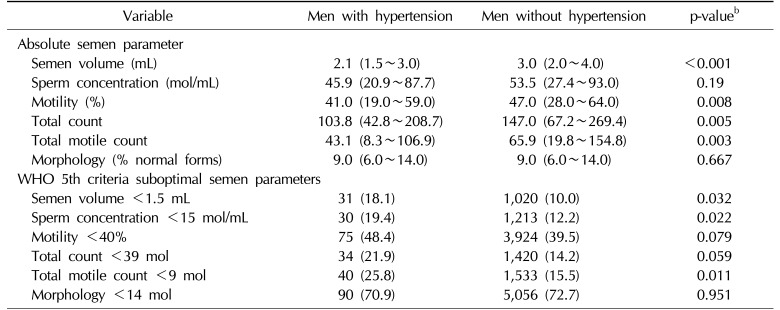
Table 2
Semen parameters and individual classes of medications
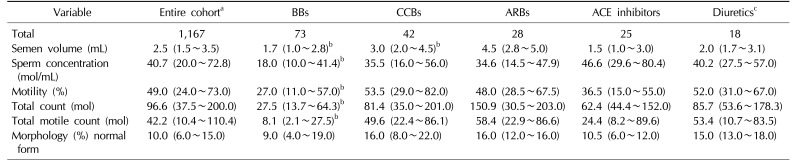
Values are presented as number only or median (interquartile range).
BBs: beta-blockers, CCBs: calcium channel-blockers, ARBs: angiotensin receptor-blockers, ACE inhibitors: angiotensin converting-enzyme inhibitors.
aAnalysis confined to men for whom medication data was available.
bp<0.05.
cWilcoxon rank-sum test instead of repeated mixed model due to limited number of diuretics.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download