Congenital ichthyoses can be divided into 3 subtypes: lamellar ichthyosis, nonbullous congenital ichthyosiform erythroderma, and HI [
5]. HI is the most severe form of congenital ichthyosis. The first description of HI was made in 1750 by Reverend Oliver Hart, and the first case of antenatal diagnosis was reported in 1983 [
6]. The overall incidence of HI is 1 in 300,000 births [
78]. HI is a congenital epidermal disorder that shows abnormal and diffuse hyperkeratosis and loss of the protective skin barrier. In humans, normal cornification of the skin begins between 14 and 16 weeks of gestation. The ABCA12 gene is essential for providing instructions on how to make a protein that is required for normal skin cell development. This protein plays an important role in the transport of lipids in the epidermis. Mutations in ABCA12 gene prevent the cell from making the ABCA12 protein. A loss of function in the ABCA12 protein disrupts the normal development of the epidermis. This subsequently results in extreme thickening of the keratin layer of the skin and the formation of hard scales [
9]. Most of affected neonates die within hours or days after birth due to sepsis, electrolyte imbalance, or mechanical restriction of breathing [
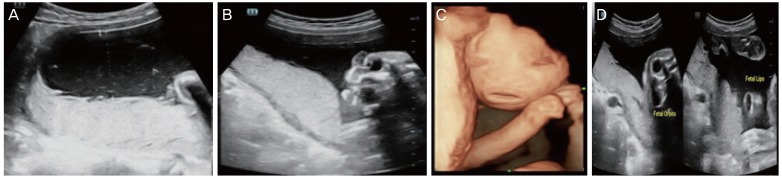
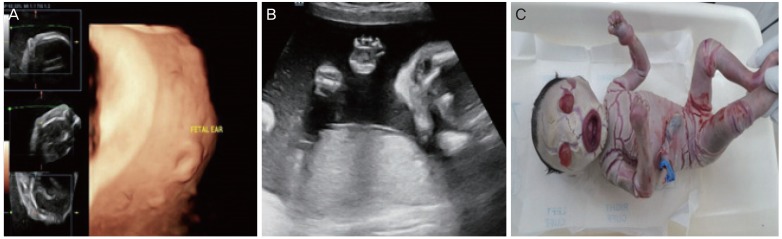
10] secondary to restricted chest expansion and prematurity. Thickened, cracked skin leads to impaired temperature regulation and increased risk of infection. The most common sonographic features observed in HI are a large open mouth, a flat nose, ectropion, short feet, and abnormal limb position. Intrauterine growth restriction, polyhydramnios/oligohydramnios, increased echogenicity of amniotic fluid, and floating membranes may also be associated with HI. Because eclabium and ectropion manifest in the third trimester, a diagnosis of HI based solely on these findings occurs too late. However, achieving an early sonographic diagnosis of HI is difficult. Short feet may be an early marker for HI, especially in families with a history of siblings affected by HI [
1112]. The other early feature is fixed, extremely hyperflexed toes, as described in a study by Vijayaraghavan et al. [
13]. A literature review found that the earliest diagnosis by 3D occurred at 22 weeks in cases with a previous history, whereas in unsuspected cases the earliest diagnosis was made at 30 weeks [
14]. Differential diagnoses include arthrogryposis, aplasia cutis, Gaucher disease (collodion baby), Sjogren-Larsson syndrome, Conradi-Hunermann-Happle syndrome, Neu Laxova syndrome, and trichothiodystrophy [
15]. HI is an autosomal recessive condition, and parents who have already had an affected child have a 25% risk of recurrence in each pregnancy [
16]. Consequently, the high recurrence rate allows a prenatal diagnosis to be performed for families at risk. HI can be diagnosed using either amniocentesis or CVS. Both of these procedures are used to obtain a DNA sample from the fetus to look for mutations in the ABCA12 gene. However, ultrasonography can also diagnose HI, and this is particularly important as it allows antenatal diagnosis even in cases with no family history of the disease. Early sonographic diagnosis is difficult, and most of cases are diagnosed in the third trimester. Hence, prenatal ultrasonography can establish the diagnosis of HI in the early third trimester.
HI is a rare autosomal recessive disorder with high subsequent recurrence. DNA analysis for ABCA12 mutations can be offered to suspected cases and to families who have previously been affected. The prenatal ultrasonography findings suggestive of a harlequin fetus are atypical facial dysmorphism, a large open mouth, the absence of typical nasal morphology, partitioned cystic formations in front of the eyes, the absence of typical ear morphology, thick skin, minimal fetal movement with stiff limbs in a semi-flexed position, limb anomalies with hypoplastic fingers, toes, and short phalanges, clubfoot, shriveled hands that do not open, hyperechogenic amniotic fluid, and an absence of associated visceral anomalies. 3D imaging is essential for understanding the 2D images and enabling diagnosis of HI. The characteristic features in prenatal ultrasonography tend to appear late, so scans should be repeated even when the second trimester anatomy scan is normal. In addition, these scans can help in situations when a DNA diagnosis is unavailable.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download