Introduction
The prevalence of breast cancer is high, with a mortality rate of 23% among female cancer patients and 14% among all cancer patients worldwide [
1]. Current therapies for patients with breast cancer include surgery, chemotherapy, and radiation therapy; surgery is considered the definitive treatment. However, surgery is not recommended to all breast cancer patients, and traditional chemotherapies have multiple side effects, including the emergence of resistant cells and toxic side effects. Therefore, novel therapies and treatments are being investigated [
12].
Photodynamic therapy (PDT) is a minimally invasive therapy that involves irradiation of targeted cancerous lesions [
3]. The 3 main factors involved in PDT are a photosensitizer, light, and oxygen irradiation onto the photosensitizer results in excitation, which then produces oxygen free radicals that damage the targeted cancer cells [
4]. Known photosensitizers used in PDT include porphyrin-family material, chlorine-family material, and phthalocyanines [
5]. Photofrin
®, a porphyrin derivative, was first used in Canada (1993) for the treatment of bladder cancer and has since been used for the treatment of different cancers, including esophageal and lung cancer, in the US, Japan, and Europe [
45]. Because Photofrin
® is not degraded and remains intact inside the cell for several weeks, patients who receive PDT using Photofrin
® are advised to cover their skin, apply sunblock, and wear sunglasses in order to avoid exposure to sunlight [
5]. Photolon
® is a water-soluble material synthesized by mixing the chlorine derivative e6 with polyvinylpyrrolidone [
6]. Photolon
® accumulates within the cancer cell approximately 1 hour after intravenous injection and most of the Photolon
® in the body is excreted after 12 hours. This allows patients to resume normal activity within a relatively short period after PDT [
7]. However, low levels of photoreaction have been reported following the injection of photosensitizer due to low solubility and potential interaction with other biomolecules. Consequently, different attempts have been made (i.e., development of novel drugs, combination therapy with chemotherapeutic agents) to improve treatment effectiveness [
348].
Lasers are often used as a light energy source to activate the photosensitizer. The laser used in PDT has a low power output, and a diode laser is commonly used due to its low cost and convenient management [
9]. The wavelength of the laser used in PDT differs depending on the photosensitizer; for porphyrin- or chlorine-family material, wavelengths of 600–700 nm (red light) are used, since they are activated within this range of wavelengths [
10].
Cisplatin, also known as cis-platinum or cis-diamminedichloroplatinum (II), is a broad-spectrum anticancer drug; it is a chemotherapeutic agent used for the treatment of different types of cancers [
11]. Cisplatin is effective against different cancer types, including carcinoma, sarcoma, and embryoma. It is widely used for the treatment of various cancer types including cancers of the head and neck, breast, lungs, colon, bladder, and genital organs [
12]. Nevertheless, the emergence of cells that are resistant to cisplatin [
13] and the side effects associated with the drug (i.e., renal damage, gastric disorders, and auditory nerve damage) have limited its use in humans [
14]. Due to its side effects and associated resistance, cisplatin-based chemotherapy is usually used in combination with different chemotherapeutic agents or other treatment methods, including PDT [
15]. Ali et al. [
16] utilized a myosarcoma cell line and reported that a combination of cisplatin and PDT resulted in increased anticancer activity, while Wei et al. [
17] demonstrated that a combination of cisplatin and 5-aminolevulinic acid in a cervical cancer cell line (HeLa) increased the effectiveness of PDT. This study aimed to assess the effect of cisplatin on PDT in breast cancer using nude mice injected with a breast cancer cell line line (experimental mammary tumour-6 [EMT6] cells) to create a breast tumor-bearing mouse model. Mice were injected with Photolon
® and cisplatin, and then subjected to PDT. Different treatment outcomes were assessed, including tumor volume, lipid peroxidation in tumor tissue, and changes in the expression of genes associated with the inflammatory response.
Materials and methods
1. Experimental materials
Cisplatin was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), while fetal bovine serum (FBS), streptomycin, penicillin, and Dulbeco's Modified Eagle's Media (DMEM) were obtained from Gibco BRL (New York, NY, USA). The thiobarbituric acid reactive substance (TBARS) Assay Kit was purchased from KOMA Biotech Inc. (Seoul, Korea), and Photolon® was purchased from Belmedpreparaty (Minsk, Belarus). The EMT6 cell line (Mus musculus breast carcinoma cells) was purchased from American Type Culture Collection.
2. Establishment of a breast cancer-bearing mouse model
The experimental animals used in this study were generated from the nude mouse strain KSN/Slc at 6 weeks of age, purchased from the Central Lab Animal Inc. (Seoul, Korea). Mice were reared under a 12-hour day-night cycle, temperature of 20±2°C, and relative humidity of 60±5%. Animal experiments were performed according to the animal research ethics protocol, with approval from the Animal Research Ethics Board of Chosun University (approval number: CIACUC 2015-A0034). The experimental mice underwent a 1-week adaptation period to the new environment prior to the induction of breast cancer. Breast cancer cells (EMT6) were cultured in DMEM containing 10% FBS, streptomycin (100 U/mL), and penicillin (100 U/mL), in a CO2 incubator at 37°C. Cultured murine breast cancer cells were harvested and resuspended in PBS, and 0.2 mL (2×105 cells) of this mixture was injected subcutaneously in the back of a nude mouse. After injection, the mice were monitored for potential tumor development, and the size of the tumor was measured using a digital caliper (Mitutoyo Korea, Busan, Korea). Mice that developed up to 9-mm tumors 10 days after the injection were used in the experiment.
3. Photodynamic therapy
Mice with induced tumors (size: ≥9 mm) were divided into 4 groups, with 10 mice in each group: control group, cisplatin group, PDT group, and combination (cisplatin+PDT) group. Cisplatin was diluted in normal saline solution and injected into the abdominal cavity of each mouse (3 mg/kg mouse BW) 1 hour prior to Photolon® injection. Similarly, Photolon® was diluted in normal saline solution and injected into the abdominal cavity of each mouse (2.5 mg/kg mouse BW). Finally, PDT was performed 2 hours after Photolon® injection by emitting a non-thermal laser light (Ceralas™Diode Laser 632 System; BioLitec, Germany, 660 nm, 80 J/cm2) onto the cancerous lesion.
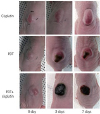
Tumor mass was assessed in 5 mice from each group before and after PDT (in different time points) until day 7, and images were obtained. Tumor size was measured using a digital caliper, and the volume was calculated using the following equation:tumor volume (mm3)=(width2×length)/2
4. Measurement of thiobarbituric acid reactive substance within the tumor tissue
Twenty-four hours after PDT, 5 mice from each group were sacrificed and the tumor tissues were resected. Part of the resected tumor was used as to determine the amount of TBARS, which is a product of lipid peroxidation.
To prepare samples for TBARS measurement, 0.3 g of tissue was resected and mixed with 3 mL of 0.1 M phosphate buffer (pH 7.0) prior to homogenization with a polytrone homogenizer. The amount of TBARS was measured using the OxiSelect™ TBARS Assay Kit (KOMA Biotech Inc.) according to the manufacturer's protocol.
5. Transcriptome profiling of the tumor tissues
Part of the tumor tissue was resected, and total RNA was extracted using TRIzol
® RNA Isolation Reagent (Life Technologies, Carlsbad, CA, USA). Using the RNA extracted from the tumor tissue, an mRNA sequencing library was created using the TruSeq stranded mRNA sample preparation kit (Illumina, San Diego, CA, USA), according to the manufacturer's protocol. The library was subsequently analyzed using an Agilent DNA High Sensitivity Kit (Agilent, Santa Clara, CA, USA) and BioAnalyzer 2100. Ultimately, the library was sequenced on the Illumina HiSeq 2500 platform for subsequent RNA sequencing analysis. To generate a cDNA library from the tumor tissues, clusters of cDNA libraries were created using TruSeq flow cell, and sequences were analyzed using the TruSeq 200 Cycle SBS kit (Illumina), which produced 100-bp end reads. The sequencing results of cDNA libraries generated by Illumina HiSeq 2500 were comparatively analyzed with the information stored in FASTQ format. Gene sets were analyzed using the Functional Annotation Tool from DAVID Bioinformatics Resources 6.7, NIH (
http://david.abcc.ncifcrf.gov) [
18]. Differences in gene expression among the experimental groups were assessed using the fold-change false discovery rate, calculated using reads per kilobase per million mapped reads, with
P<0.05 denoting statistically significance.
6. Statistical analyses
All measurements, except for RNA-seq results, were represented by mean±standard deviation, and the experimental outcomes were analyzed with analysis of variance using Statistical Package for the Social Science Ver. 12.0 (SPSS Inc., Chicago, IL, USA). Significance among samples was assessed using Duncan's multiple range test, at P<0.05.
Discussion
In the present study, experiments using a breast cancer mouse model were performed to evaluate the effects of cisplatin combined with PDT using Photolon
®. In the combination group, the area of blackening on the surface of the tumor was larger, and the tumor was smaller compared with the PDT group. PDT induces localized damage to irradiated lesions, and damage to the cancer cells and vascular system within the tumor tissue can induce necrosis of the tumor tissue. Darkening of the skin that covers the tumor tissue indicates necrosis; therefore, the observed color change (to black) of the skin that covers the cancerous lesion after PDT may indicate that the tumor tissue had undergone necrosis. Following injection, photosensitizers accumulate within lysosomes or mitochondria, and irradiation with a laser causes lysosomal hydrolase to leak into the cytoplasm, resulting in cell necrosis [
19]. Furthermore, PDT causes damage to vascular endothelial cells, resulting in thrombus formation and vessel blockade, and thus inducing secondary necrosis of the tumor [
20].
The change in skin color in the PDT group was only observed in the middle part of the tumor tissue; this finding indicates that tissue necrosis only occurred in a specific region of the tumor. Conversely, the combination group exhibited a change in the color in the skin across the entire tumor surface, suggesting that tissue necrosis occurred throughout the tumor. These experimental findings suggest that cisplatin provides additive antitumor effects to PDT using Photolon®. Moreover, since the cisplatin group exhibited no tumor growth or changes in skin color, the amount of cisplatin injected in this study (3 mg/kg mouse BW) had no or minimal effects on the growth or necrosis of cancer cells.
Cancer cell proliferation increases tumor volume. In this study, tumor volume in the control group increased by 21% and 135% on days 3 and 7 after PDT, respectively, demonstrating that the tumor is continuously growing. Mice in the cisplatin group had a similar rate of tumor growth compared with those in the normal group; therefore, cisplatin injection (3 mg/kg mouse BW) appeared to have no effect on the suppression of tumor growth. Tumor volume in the PDT group, compared with that before treatment (day 0), increased by 67% and 74% on days 3 and 7, respectively. Additionally, the rate of tumor growth on day 3 was greater in the PDT group than in the control group. One explanation for this observation is the inflammatory response following photodynamic reaction. Nonetheless, by day 7, the rate of tumor growth was lower in the PDT group compared with the control group. Tumor volume in the combination (cisplatin+PDT) group, compared with that before treatment (day 0), was increased by 77% and 52% on days 3 and 7, respectively. On day 3, the rate of tumor growth in the combination group was greater than that in the PDT group, which may have been caused by the greater inflammatory response following the photodynamic reaction. On day 7, both groups exhibited a decrease in tumor volume, further indicating that cisplatin injection induces an additive effect and maximizes the effectiveness of PDT using Photolon®.
Following injection of cisplatin into the abdominal cavity of mice, the concentration of cisplatin within the tissue reaches maximal levels within 15 minutes and lasts for 0.5–4 hours [
21]. In this study, there was a 3-hour interval between cisplatin injection and laser irradiation, and the latter likely occurred when the concentration of cisplatin in the tissue was maximal. The lethal dose of cisplatin in mouse is ≥20 mg/kg [
21]; however, the concentration of cisplatin used in this study was 3 mg/kg, which is less than that typically used (5 mg/kg). The tumor size in the combination group was lesser than that in the PDT group. This change was probably a result of the interaction between cisplatin and activated Photolon
®, which induced an additive effect on tumor suppression. Ali et al. [
16] treated a myosarcoma cell line with PDT, using a phthalocyanine-family photosensitizer, aluminum phthalocyanine tetrasulfonate chloride and demonstrated that injection of low-dose cisplatin resulted in increased anticancer activity. In addition, Ge et al. [
8] suggested that cisplatin increased anticancer activity in a colorectal cancer cell treated with PDT using a photosensitizer called Photogem
®. In this study, injection of cisplatin increased the effectiveness of PDT using Photolon
® in a breast cancer mouse model, producing a similar outcome as those reported in previous studies.
The therapeutic effect of PDT depends on the photosensitizer producing free radicals through a photodynamic reaction. When the photosensitizer is irradiated, the photodynamic reaction occurs and produces reactive oxygen species (ROS) [
22]. As cisplatin increases ROS synthesis [
23], cisplatin combined with PDT may promote ROS synthesis. In turn, ROS induces lipid peroxidation. In the present study, the levels of lipid peroxidation products in the tumor tissue was measured by assessing the amount of TBARS. The amount of TBARS in the tumors of PDT group was increased by up to 70% compared with that in the control group, indicating an increased amount of lipid peroxidation product from PDT. Furthermore, the amount of TBARS in the tumors of the combination (cisplatin+PDT) group was greater than that in the PDT group (up to 47%), further supporting the hypothesis that a combination of cisplatin and PDT increased the oxidative damage of cancer cells. Localized inflammation following PDT is a key process of this anticancer therapy; this response involves multiple different factors, including vasoactive substances, the complement system, acute-phase proteins, proteinases, peroxidases, ROS, white blood cell chemoattractants, cytokines, growth factors, and other immune response mediators [
24].
To examine the effect of cisplatin on the expression of inflammatory response-related genes within tumor tissues subject to PDT, the tumor tissue transcriptome was analyzed with mRNA-sequencing on day 1 after PDT and the outcomes from the PDT group and combination (cisplatin+PDT) group were compared. Inflammatory response-related genes with a log2(FC) value ≥ 1 and P<0.05 included CL2, CL7, CL12, XCL1, XCL2, XCL5, XCLl0, IL-1β, and IL-6. Conversely, Fn1 was the only gene with a log2(FC) ≤ –1 and P<0.05.
CL2 and
CL7 are secreted from macrophages and exhibit chemotaxis towards monocytes [
2526].
CL12 is secreted from macrophages but exhibits chemotaxis towards monocytes, eosinophils, and lymphocytes [
27].
XCL2 is secreted from monocytes and macrophages, exhibiting chemotaxis towards polymorphonuclear leukocyte [
28], and
XCL5 is secreted from endothelial cell or neutrophils during the acute inflammatory response to activate neutrophils [
29].
XCL10 is secreted from monocytes or endothelial cells, and exhibits chemotaxis towards monocytes and macrophages [
30].
IL-1β and
IL-6 are secreted from activated macrophages to induce an inflammatory response [
31].
Fn1 is involved in intracellular connections within the tissue, and promotes repair of tissue damage [
32]. Compared with the PDT group, the combination group exhibited increased expression of genes involved in chemotaxis (i.e.,
CL2, CL7, CL12, XCL1, XCL2, XCL5, and
XCLl0) to recruit and activate cells involved in the inflammatory response (i.e., monocytes, eosinophils, neutrophils, and macrophages). In addition, the levels of
IL-1β and
IL-6 were increased, which promote inflammation. Together, the increased expression of these genes is thought to drive further inflammation in the tumor tissue. Conversely, the expression of
Fn1, which suppresses the immune response and promotes wound healing, was decreased. The increased inflammatory response in the combination group, compared with the PDT group, is thought to result in an increased anticancer effect.
Overall, these experiments demonstrated that, in a breast cancer mouse model treated using PDT with Photolon®, a combination of PDT and low-dose cisplatin resulted in an increase cancer cell necrosis, oxidative damage in the tumor tissue, and inflammation within the tumor tissues. Therefore, we suggest that cisplatin treatment combined with PDT may bring additive therapeutic effects for the treatment of cancerous lesions, although additional future studies are needed to confirm these findings.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download