This article has been corrected. See "Erratum: Correction of Figure: Fertility preservation for adolescent and young adult cancer patients in Japan" in Volume 62 on page 146.
Abstract
Adolescent and young adult (AYA) patients are generally defined as being from 15 to 39 years old. For preservation of fertility in AYA cancer patients, the best-known guideline in this field was released by the American Society of Clinical Oncology (ASCO) in 2006. However, the ASCO guideline is not necessarily applicable to Japanese cancer patients. The Japan Society for Fertility Preservation (JSFP) was formed in 2012, and a system and guideline for fertility preservation in Japanese AYA cancer patients plus children was released in July 2017. According to this guideline, patients should receive psychological and social support from health care providers such as doctors, nurses, psychologists, pharmacists, and social workers. In 2013, the American Society for Reproductive Medicine stated that freezing oocytes is a method that has passed beyond the research stage. However, freezing ovarian tissue is still a research procedure. While slow freezing of ovarian tissue is generally performed, rapid freezing (vitrification) is more popular in Japan. We have developed a new closed technique for ovarian tissue cryopreservation. It has been suggested that optical coherence tomography might be applied clinically to measure the true ovarian reserve and localize follicles in patients undergoing ovarian tissue transplantation. Combining gonadotropin-releasing hormone agonist therapy with anticancer agents might be useful for ovarian protection and it is expected that discussion of such combined treatment will continue in the future. This article outlines practical methods of fertility preservation using assisted reproductive techniques for AYA cancer patients in Japan.
The adolescent and young adult (AYA) generation means people in adolescent and young adulthood and is defined as a generation from 15 to 39 years old. The study about cancer in this generation is defined as adolescent and young adult oncology (AYAO) by 2011 National Cancer Institute (NCI) Cancer Bulletin. There are various definitions in the AYA generation. The Surveillance, Epidemiology, and End Results (SEER), NCI and National Comprehensive Cancer Network (NCCN) define the ages from 15 to 29 years old and from 15 to 39 years old, respectively. At SEER AYA Monograph 2006 [1] reported that cancer patients between the ages of 15 and 29 are only 2–3% of all cancer patients, but 2.7 times those of cancer patients under 15 years of age. According to Clinical Practice Guidelines, Adolescent Young Adult Oncology and the SEER Cancer Statistics Review 2009–2013 in NIH (the NCI: SEER program), they reported that the most common cause of death to women in AYA generation excluding accidents and suicide and the most common in the order of cancer incidence (number per 100,000 people) is the thyroid cancer, breast cancer, cervical carcinoma, malignant melanoma, and colorectal cancer to women and gonadal cell tumor, malignant melanoma, non-Hodgkin's lymphoma, colorectal cancer, thyroid cancer to men [23]. In Japan, according to the 2015 cancer incidence rate (2011) based on areas and ages reported by Cancer Counseling Information Center of the National Cancer Center, the highest rates in order for female are thyroid cancer, leukemia, ovarian cancer at the ages of 20 to 24 years old, uterine cervical cancer (excluding carcinoma in situ), breast (not including carcinoma in situ), thyroid carcinoma at the age of 25 to 30 years old, uterine cervical cancer (not including carcinoma in situ) and thyroid carcinoma at the age of 30 to 34, and then breast (not including carcinoma in situ), uterine cervical cancer (not including carcinoma in situ) and thyroid carcinoma at the age of 35 to 39 years of age.
For male, leukemia, thyroid cancer, malignant lymphoma at the age of 20 to 24, colorectal cancer, leukemia, malignant lymphoma at the age of 25 to 30, colorectal cancer, malignant lymphoma, gastric cancer at the age of 30 to 34, and colorectal cancer, colon cancer, gastric cancer at the age of 35 to 39 years old [4]. In fact, fertility preservation therapy to be prioritized by cancer type may be different in cancer/reproductive medicine (reproductive care for AYA generation cancer patients: Oncofertility). This paper outlines the practical fertility preservation therapy using reproductive assisted technology for AYA generation cancer patients.
In 2006, guidelines on fertility preservation for young cancer patients were presented from the American Society of Clinical Oncology (ASCO), which was later revised in 2013 [56]. ASCO, in collaboration with the American Society for Reproductive Medicine (ASRM), published the guideline on fertility preservation therapy for cancer patients for the first time in the world in 2006. This ASCO 2006 presents guidelines on pregnancy rate, birth rate, and in vitro fertilization (IVF) success rate in papers meeting criteria among other report papers from 1985 to 2005, and it also states that the information about concerning the possibility of fertility decline by cancer treatment should be informed to cancer patients at all reproductive age. In addition, cancer therapists should present problems on fertility to patients in the early stages of diagnosis and emphasize that cooperation with doctors specializing in reproductive medicine is important. The change in the revised version in 2013 was that all providers (such as doctors, nurses, social workers, psychologists, etc.) involved in fertility preservation treatment be referred to as healthcare providers and the needs to respond to patients by the entire healthcare provider is clearly indicated and they are responsible for providing information to cancer patients regarding the possibility of deteriorating fertility.
On the other hand, in Japan, the Japanese Society for Reproductive Medicine “Guidelines on the Freezing and Preservation of Unfertilized Oocyte and Ovarian Tissue” was published in 2013, and the Japan Society of Obstetrics and Gynecology issued “Observation on Collection, Freezing and Preservation of Ovarian Tissue” in April 2014 and was partially revised in 2016. However, there are no comprehensive guidelines on fertility preservation for various cancers in Japan. With a view to improving the quality of life of cancer survivors, cancer therapists need to reaffirm the importance of fertility preservation for AYA generation cancer patients. Depending on the type and progress of cancer, cancer therapy is given as a top priority, and there are many circumstances in which we are forced to abandon future pregnancy and childbirth. Therefore, the Japan Cancer Therapeutics Association started making guidelines focusing on cancer treatment from October 2015, carefully considering fertility preservation for AYA cancer patients and children (<15 years old) with cancer. In July 2017, guidelines on fertility preservation for young cancer patients (children and AYA; CAYA) were published by the Japan Society of Clinical Oncology [7].
Oocyte cryopreservation is one of fertility preservation treatments for young cancer patients who do not have a partner, and ASRM has said that this method is not a research stage in 2013 [8]. In oocyte freezing, patients will need to receive hormone preparations as they will store mature oocytes (second meiotic metaphase oocytes). And since collection of oocytes depends on the menstrual cycle, it is common that it takes at least 2 weeks before harvesting. The patient will receive an injection of follicle stimulating hormone (FSH) formulation soon after menstruation, but injections around 10 days are needed to raise follicles and mature oocytes. When the follicle develops thereafter, the patient receives human chorionic gonadotropin (hCG) or gonadotropin-releasing hormone (GnRH) agonist (including nasal spray) preparation for oocyte acquisition, and oocyte collection is performed about 36 hours later. Because it takes time to collect oocytes like this and the timing of oocyte collection depends on the menstrual cycle when a cancer patient visits obstetrics and gynecology department, it is also assumed that freezing cannot be done when no time to start treatment. However, in recent years, the random start method [9], which is an attempt to enforce oocyte production irrespective of the patient's menstrual cycle at the time of visit to obstetrics and gynecology, was devised and it became possible to have treatment for oocyte acquisition without waiting for the next cycle menstruation. ASRM states that pregnancy rate in oocyte freezing depends on age. Among them, quoting a large-scale study on the guidelines, it reports that the pregnancy rate per embryo transplant is 27.7% below 34 years old, 21.4% from 35 to 38 years old, 21.4% from 38 years old, and the pregnancy rate per embryo transplant is 48.6% below 34 years, 24.1% from 35 to 37 years old, 23.3% from 38 to 40 years old, and 22.2% from 41 to 43 years old [8]. When multiple mature oocytes are collected, female hormone levels (estradiol values) increase due to ovulation stimulation by FSH, so that more attention must be paid to adverse effects, proliferation to cancer cells by estradiol values being caused for patients with hormone receptor positive breast cancer and endometrial cancer. Therefore, when oocytes are harvested, hormone receptive positive breast cancer may select a treatment method to recover oocytes by ovarian stimulation with aromatase inhibitor combination for the purpose of suppressing increase in estradiol value [9]. Reddy and colleagues [10] classified the number of acquired oocytes, the number of matured oocytes, the number of frozen embryos, the estradiol value, etc. among the 2 groups in GnRH agonist and hCG ovulation determination medicine in fertility preservation treatment combined with an aromatase inhibitor. The results are 14.1±6.6 and 12.4±8.6, respectively, the number of acquired eggs (individual) 10.5±5.1 and 7.7±5.3, the number of frozen embryos (pieces) 7.7±4.2 and 5.4±73.8, the estradiol value (pg/mL) 564.7±313.2 and 529.7±400.6, indicating that the increase in estradiol level is sufficiently suppressed. Oktay and colleagues [11] obtained 25 live births as a result of carrying out 40 embryo transplantations to 33 patients in IVF with aromatase inhibitor combination, but it was reported that none of children were observed fetal malformation etc.
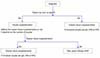
On the other hand, if a cancer patient has a partner, fertilization is frozen by embryo with the oocyte collected using the partner's sperm. There are two kinds of fertilization methods: Conventional IVF and intracytoplasmic sperm injection (ICSI). Conventional IVF is a method of removing waste from semen after ejaculation on the collected mature oocytes, culturing the sperm together with oocytes in the culture solution, and confirming fertilization the next day. On the other hand, ICSI is a method of placing one sperm into a matured oocyte under a microscope against cultured oocytes, culturing until the next day and confirming fertilization. Although the summary of oocyte freezing and embryo (fertilized oocytes) freezing has been shown above, in principle the oocytes collection involves transvaginal operation, so there are cases where it cannot be performed in patients with AYA generation female cancer (Fig. 1). The Japan Society of Obstetrics and Gynecology shows “Views on unfertilized oocytes, embryos (fertilized oocytes) and ovarian tissues freezing and preservation by medical adaptation” in June 2016, and in Japan when oocytes or fertilized oocytes for AYA generation cancer patient are frozen, it is required for physician to notify the Japan Society of Obstetrics and Gynecology [12]. The following is excerpted from the sentence: “By conducting surgical therapy, chemotherapy, radiation therapy, etc. for women who are suffering from malignant tumor etc. (hereinafter called the original disease) for the purpose of treating the original disease,” if ovarian function decreases before experiencing pregnancy/childbirth, and as a result it is predicted that fertility will be lost, unfertilized oocytes are collected based on the intention of the patient as a method of preserving fertility. It is considered that this method is a medical practice as a part of countermeasures for adverse reactions occurring in the treatment of the original disease, so although there is no desire for childbirth at the time of receiving the treatment, it is necessary to acknowledge it as a medical practice if she wishes, but due to the influence of the implementation of this rule on the prognosis of the original disease, the possibility of pregnancy by the preserved oocyte in the future and its safety, since there are many things that are not clear yet, it is important that the patients make their own decisions by being provided sufficient information. This rule is to carry out IVF/embryo transplantation and ICSI and it must be conducted in accordance with the “Observations on IVF and embryo transplant” and the “Observations on microscopic insemination” of the Japan Society of Obstetrics and Gynecology. Furthermore, since this rule encompasses medical, ethical and social problems that are different from ordinary reproductive support medicine (assisted reproductive technology; ART), it needs to be done with the following points in mind. The collection, freezing and preservation of ovarian tissue performed for the same purpose belongs to the same medical practice as in the case of unfertilized oocyte, and this view is basically applied, considering that it is included in this rule [12].
Freezing ovarian tissue for AYA cancer patients was first reported by Oktay and Karlikaya in 2000 [13] and the firstborn baby acquisition was reported by Donnez et al. [14] in 2004. According to the report of von Wolff et al. [15] in 2015, they reported that more than 1,000 cases of ovarian tissue freezing are performed in Europe, and this technology is becoming generalized, especially in Europe. However, ASRM states that ovarian tissue freezing is not an established technique but an experimentally practiced technique [16]. In ovarian tissue freezing, in principle, one ovary is removed by laparoscopic surgery and the ovary cortex is stored frozen outside the body. Therefore, it is not a technique that depends on the patient's menstrual cycle, but it can apply to all AYA generation cancer patients scheduled to undergo cancer treatment with sudden ovarian toxicity. So far, there have been 86 live births after ovarian tissue freezing worldwide [17]. The vast majority of the live births have been achieved after slow freezing of ovarian tissue, although there have been 3 live births after ovarian tissue vitrification in Japan. Rapid freezing of ovarian tissue (vitrification) is relatively popular in Japan, where there have been over 1,000 cases of ovarian tissue cryopreservation by slow freezing and also over 250 cases of ovarian tissue vitrification for fertility preservation. A total of 32 centers can perform ovarian tissue cryopreservation in Japan and are members of the JSFP. Several articles concerning ovarian tissue vitrification have been published [181920]. In August 2017, Shi and colleagues [21] compared vitrification with slow freezing of human ovarian tissue, and they concluded that vitrification may be more effective than slow freezing, with fewer primordial follicular DNA strand breaks and better preservation of stromal cells. At the ASRM meeting in 2017, we reported a comparison of follicle survival and DNA damage after vitrification versus slow freezing of human ovarian tissue. The following parameters were compared: the primordial follicle density, γH2AX-positive primordial follicle percentage, AC3 positive primordial follicle percentage, non-apoptotic primordial follicle density, primary follicle density, γH2AX-positive primary follicle percentage, AC3-positive primary follicle percentage, and non-apoptotic primary follicle density. AC3-positive primordial follicles were 17.5±2.3% at baseline, 20.1±2.7 after slow freezing, and 12.8±3.1% after vitrification, showing a statistically significant difference between slow freezing and vitrification (P=0.06, unpublished observations). Based on the impact on primordial follicle survival and DNA damage after thawing of frozen tissue and culture, there may be no major differences between slow freezing and vitrification. It seems that fewer apoptotic follicles survive after vitrification, but whether this has any impact on final non-apoptotic follicle density or survival in culture is unclear [22].
We have developed a closed device for ovarian tissue vitrification and we have performed comparison of open and closed methods for vitrification. Our results showed that the closed method had similar or better efficacy compared to open vitrification of human ovarian tissue. There was a higher primordial follicle survival rate after 96 hours of culture with closed vitrification, but the mechanism needs to be explored further. It has been shown that the open method has similar efficacy with slow freezing of ovarian tissue. Accordingly, the closed method should become the standard method of ovarian tissue cryopreservation [23]. Except for histological examination, there are currently no suitable techniques for the detection and identification of primordial follicles in patients with primary ovarian insufficiency who have undetectable anti-mullerian hormone levels. The ability to locate and quantify follicles in unfixed ovarian cortex strips would be useful in patients who are undergoing ovarian tissue transplantation. Optical coherence tomography (OCT) is a well-established high-resolution imaging technique commonly used in biomedicine that does not require tissue fixation, and it has been suggested that OCT might be employed to measure the true ovarian reserve and localize follicles [24]. However, ovarian cancer, leukemia, etc., in which cancer cells are expected to be present in the ovarian tissue, are out of the indication. That is, in the event that cancer cells are contained in frozen and thawed ovarian tissue pieces, there is a possibility that cancer cells may be transferred to themselves again by their own ovarian tissue after remission. This is called minimal residual disease (MRD). There have been several reports since MRD is reported by Dolmans et al. [2526272829]. According to the report of Greve et al. [26] in 2012, the ovaries of 4 patients with leukemia and lymphocytic leukemia who had positive cancer cells in ovarian tissue by quantitative reverse transcription polymerase chain reaction (RT-qPCR) were observed in immunodeficient mice for 20 weeks after transplantation. As a result, it was reported that no tumor was formed. There is a problem with the accuracy of RT-qPCR, and the investigation on detection of MRD in ovarian tissue is continuing in the research. Therefore, since there is no established method for diagnosing MRD at this stage, there is a need to carefully study the adaptation of this technique to patients with a high possibility of the presence of cancer cells, especially in the ovary. In addition, the birth rate for ovarian transplantation by ovarian tissue / freezing has been reported to be 25% [29], and in the report on the clinical application of this technology in accordance with Edinburgh criteria of Wallace. Ovarian tissue freezing/transplantation safety and effectiveness have been shown [30].
By the way, when the ovarian tissue is frozen, ovarian medulla is removed in the culture medium, and only the ovary cortex where the primordial follicle is present is immersed in the cryoprotective agent to carry out the freezing of the ovarian tissue. In this process, when there is a clearly developed follicle in the ovarian tissue, ovarian cortex is damaged by the formation of ice crystals in the follicular fluid [31]. Therefore, in general, in order to prevent the formation of ice crystals, the follicles that grew in the ovarian tissue are aspirated by injection needle. At the time of removal medulla and the culture solution upon aspiration with a stereoscopic microscope, premature oocytes in culture broth in a relatively frequent time can be confirmed comparatively and frequently. Techniques for culturing immature oocytes in these cultures in vitro and cryopreserving them as mature oocytes are called combined procedure [32]. That is, it is a method of combining ovarian tissue freezing and simultaneous oocyte freezing. Because most of the oocytes in these cultures are in immature state, mature oocytes will be obtained by immature oocyte in vitro culture (in vitro maturation; IVM) or premature egg growth (in vitro follicle growth; IVFG). Xiao and colleagues [33] reported that they acquired mature oocytes at a high rate in culture method using alginate beans in 150 μm immature follicles as IVFG, fertilized the mature oocytes, and reached the blastocyst.
GnRH analog is a fertility preservation therapy that minimally reduces the exposure of anticancer drugs by protecting the developmental process of primordial follicles [34]. Unfortunately, most of the studies performed to date have had various methodological problems, such as a small patient population, short study period, high proportion of patients over 40 years old, or high rate of loss to follow up. For example, the PROMISE-GIM6 study published in 2011 had a duration of only one year and most of the patients were over 40 years old [35]. When this study was extended for several years to assess the occurrence of menopause, most of the patients could not be followed [36]. In 2012, a randomized trial of the GnRH agonist triptorelin for preservation of ovarian function during neoadjuvant chemotherapy for breast cancer was published, but this was a small-scale study [37]. Furthermore, The GBG 37 ZORO study published in 2012 [38] evaluated patients with or without a menstrual cycle for only 6 months. In my opinion, the most reliable study published so far is the Prevention of Early Menopause Study (POEMS) trial. The results of the POEMS trial reported by ASCO in 2014 resulted in a very disappointing result. In ASCO, the ovarian failure rate was 22% and 8% in the standard treatment group and the GnRH analog administration group respectively 2 years after the trial registration and it was suggested the possibility of ovarian protection of GnRH analogue by anticancer drug. However, the authors concluded in a report in the NEJM magazine in 2016 that “We lost the reliability of the results of the POEMS exam because of some missing data” [39]. Meanwhile, Demeestere and colleagues [40] reported on the results of a randomized controlled trial with a median follow-up period of 5 years. As a result, the incidence of premature ovarian failure did not change in the GnRH analog administration group compared with the non-administered group. In 2016, Munhoz and colleagues [41] confirmed research results from January 1975 to March 2015 in the PubMed, SCOPUS and Cochrane databases, and further examined the meta-analysis results from ASCO's 1995 to 2014, San Antonio Breast Cancer Symposium 2009 to 2014 that also confirmed the abstract indicate that there is a possibility of ovarian protection by GnRH analogue against early breast cancer. However, Oktay and Turan [42] pointed out that there is a problem with the method of extracting meta-analysis as an Invited commentary for this paper. Primordial follicles and eggs do not originally have receptors for GnRH, and DNA damage and apoptosis in eggs in the developing follicle are induced by alkylating agents and topoisomerase inhibitors, but not in primordial follicles, resulting in ovarian dysfunction. It is said that GnRH analogs cannot protect the toxicity of anticancer drugs to primitive follicles, and further motivation for pregnancy of cancer patients assigned to the GnRH analog group is biasing, so that AYA generation cancer patient Ovarian protection by GnRH analog is not recommended as fertility preservation therapy [4042]. At this time ASCO does not recommend using GnRH analog in combination with anti-cancer drug. It is expected that the discussion whether GnRH analog will be used when anticancer drug is used will continue in future.
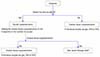
According to the AYA oncology guideline, version 2. 2014 reported by NCCN, sperm freezing (semen freezing) before anticancer treatment is the most reliable technology as a fertility preserving in AYA generation cancer-affected men and it states that this is well established as a treatment. The NCCN guideline also recommends sperm cryopreservation before treatment (Fig. 2). The reason is that sperm after anti-cancer treatment may induce genetic defect. In addition, it is reported that the original disease may induce azoospermia in Hodgkin lymphoma and testicular cancer patients [43]. Similar to the NCCN guidelines, Moss and colleagues also says that ejaculate semen is the most effective male fertility preservation therapy for AYA generation and should be collected before anticancer treatment. If ejaculation is impossible and family members and patient can be accepted, artificial ejaculation such as Penile Vibratory Stimulator (machine to promote ejaculation) or Electroejaculation under general anesthesia (electric ejaculation) can also be performed. If ejaculate semen cannot be obtained, it is said that surgically performing testicular sperm extraction (TESE) operation will collect sperm. This is because radiation therapy and anti-cancer treatment are known to greatly affect spermatogenesis [44].
Basal and clinical studies of testicular tissue freezing have been started in recent years. With current technology, it is not possible to mature spermatogonial stem cells in vitro due to IVF in the future. However, Gupta and colleagues [45] have conducted a testicular biopsy of 77 pre-adolescent boys and said that preserved tissue can contain preserved spermatogonial stem cells because they may contain spermatogonial stem cells. It is not an option to preserve fertility. In addition, Long and colleagues [46] also said that freezing testicular tissues is still at the research stage, however, it is indicated that there is adaptation to boys with pre-pubertal cancer. Although it is possible to preserve spermatogonial stem cells by freezing testicular tissues, they say that they will re-transplant after treatment and it will be cryopreserved in anticipation of sperm differentiation resuming.
A report by Dr. Donnez et al. [14] in 2004 of the young female Hodgkin's disease patient for the first time to acquire a baby by ovarian tissue freezing and ovarian transplantation after cancer therapy has become a breakthrough in this area, and a new area called Oncofertility is established by Dr. Woodruff [47]. Also in Japan, the practice of fertility preservation therapy for AYA generation cancer patients have conducted in 2012 by the JSFP (http://www.j-sfp.org/index.html), various societies and organizations have deepened interest in this area. In June 2015, the “Cancer Summit” hosted by the Ministry of Health, Labor and Welfare formulated the “Acceleration Plan for Cancer Countermeasures,” so far there has been no clear description in the Basic Plan for Promotion of Cancer Countermeasures. It is now that the aim of achieving sustainable cancer control in the future is indicated by the importance of “cancer countermeasure according to life stage such as childhood, AYA generation, mature age, elderly age.” Since “Implementation for reproductive dysfunction” was included in cancer control according to the life stage this time, it is urgent for not only doctors but also all healthcare providers including nurses, psychologists, pharmacists, etc. to construct a cancer and reproductive medical cooperative network aiming at practicing fertility preservation therapy for cancer patients in the AYA generation in Japan.
Acknowledgements
I really appreciate my friend, Ms. Reiko Koda for helping English translation and editing my English.
References
1. Bleyer A, O'Leary M, Barr R, Ries LAG. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age: including SEER incidence and survival: 1975??000. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health;2006.
2. Coccia PF, Altman J, Bhatia S, Borinstein SC, Flynn J, George S, et al. Adolescent and young adult oncology. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012; 10:1112–1150.
3. SEER cancer statistics review (CSR) 1975??013 [Internet]. Bethesda (MD): National Cancer Institute;2016. cited 2012 Oct 20. Available from: http://seer.cancer.gov/csr/1975_2013/browse_csr.php?sectionSEL=32&pageSEL=sect_32_table.01.html.
4. Foundation for Promotion of Cancer Research (JP). Cancer Statistics in Japan 2015 [Internet]. Tokyo: Foundation for Promotion of Cancer Research;2015. cited 2012 Oct 20. Available from: http://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2015_jp.html.
5. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006; 24:2917–2931.

6. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013; 31:2500–2510.

7. Japan Society of Clinical Oncology. JSCO clinical practice guidelines 2017 for fertility preservation in childhood, adolescent and young adult cancer patients. Tokyo: Japan Society of Clinical Oncology;2017. in Japanese.
8. Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013; 99:37–43.
9. Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013; 100:1673–1680.

10. Reddy J, Turan V, Bedoschi G, Moy F, Oktay K. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: an extended experience. J Assist Reprod Genet. 2014; 31:927–932.

11. Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015; 33:2424–2429.

12. Japan Society of Obstetrics and Gynecology. Views on unfertilized oocytes, embryos and ovarian tissues freezing and preservation by medical adaptation. Acta Obstet Gynaecol Jpn. 2016; 68:1470–1474.
13. Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000; 342:1919.

14. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004; 364:1405–1410.

15. von Wolff M, Dittrich R, Liebenthron J, Nawroth F, Schüring AN, Bruckner T, et al. Fertility-preservation counselling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online. 2015; 31:605–612.

16. Practice Committee of American Society for Reproductive Medicine. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014; 101:1237–1243.
17. Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017; 34:325–336.

18. Suzuki N. Ovarian tissue cryopreservation using vitrification and/or in vitro activated technology. Hum Reprod. 2015; 30:2461–2462.
19. Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015; 30:608–615.

20. Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013; 110:17474–17479.

21. Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep. 2017; 7:8538.

22. Kawahara T, Sugishita Y, Taylan E, Suzuki N, Moy F, Oktay KH. Vitrification versus slow freezing of human ovarian tissue: a comparison of follicle survival and DNA damage. Fertil Steril. 2017; 108:e56–e57.

23. Sugishita Y, Taylan E, Kawahara T, Suzuki N, Oktay KH. Comparison of open and closed devices in human ovarian tissue vitrification. Fertil Steril. 2017; 108:e172–e173.
24. Takae S, Tsukada K, Sato Y, Okamoto N, Kawahara T, Suzuki N. Accuracy and safety verification of ovarian reserve assessment technique for ovarian tissue transplantation using optical coherence tomography in mice ovary. Sci Rep. 2017; 7:43550.

25. Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010; 116:2908–2914.

26. Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sørensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012; 120:4311–4316.

27. Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013; 99:1514–1522.

28. Hoekman EJ, Smit VT, Fleming TP, Louwe LA, Fleuren GJ, Hilders CG. Searching for metastases in ovarian tissue before autotransplantation: a tailor-made approach. Fertil Steril. 2015; 103:469–477.
29. Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015; 385:506–507.

30. Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014; 15:1129–1136.

31. Sugishita Y, Hashimoto S, Hoshina M, Kawagoe Y, Yoshioka N, Takae S, et al. Ovarian tissue vitrification: ovarian tissue cryopreservation and transplantation. Ishiyakushuppan. 2017; 7:46–59. (in Japanese).
32. Takae S, Sugishita Y, Yoshioka N, Hoshina M, Horage Y, Sato Y, et al. The role of menstrual cycle phase and AMH levels in breast cancer patients whose ovarian tissue was cryopreserved for oncofertility treatment. J Assist Reprod Genet. 2015; 32:305–312.

33. Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction. 2015; 150:183–192.

34. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995; 52:365–372.
35. Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011; 306:269–276.

36. Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival, a randomized clinical trial. JAMA. 2015; 314:2632–2640.
37. Munster PN, Moore AP, Ismail-Khan R, Cox CE, Lacevic M, Gross-King M, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012; 30:533–538.

38. Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011; 29:2334–2341.

39. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015; 372:923–932.

40. Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016; 34:2568–2574.

41. Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2:65–73.
42. Oktay K, Turan V. Failure of ovarian suppression with gonadotropin-releasing hormone analogs to preserve fertility: an assessment based on the quality of evidence. JAMA Oncol. 2016; 2:74–75.
43. Coccia PF, Pappo AS, Altman J, Bhatia S, Borinstein SC, Flynn J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw. 2014; 12:21–32.

44. Moss JL, Choi AW, Fitzgerald Keeter MK, Brannigan RE. Male adolescent fertility preservation. Fertil Steril. 2016; 105:267–273.

45. Gupta AA, Donen RM, Sung L, Boydell KM, Lo KC, Stephens D, et al. Testicular biopsy for fertility preservation in prepubertal boys with cancer. Identifying preferences for procedure and reactions to disclosure practices. J Urol. 2016; 196:219–224.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download