Abstract
Objective
The objective of this study was to assess the association between women with endometriosis and risk of preterm birth.
Methods
Two reviewers independently determined all prospective cohort study, retrospective cohort study, large population based cohort study, retrospective secondary analysis, and double blinded, multicentric, observational and cohort study, placebo-controlled, randomized clinical trial published using PubMed, Medline, Korea Education and Research Information Service, and Scopus from March 1994 through February 2016 without language restrictions comparing obstetric outcomes women with endometriosis and women without endometriosis. The meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. Six studies met inclusion criteria, including 50,472 women. Among 50,472 pregnancies, 39,659 had endometriosis and 10,813 had no endometriosis. Meta-analyses were estimated with odds ratios and 95% confidence intervals using random effect analysis according to heterogeneity of studies.
Preterm birth is a cause of neonatal morbidity and future adult diseases as well, and affects 5% to 15% of all pregnancies, and its prevention is a significant healthcare topics. Preterm birth is one of the great obstetrical syndromes and is occurred by multiple causes.
Endometriosis is a chronic reproductive disease characterized by the presence of endometrial glands outside the uterus, mainly on the ovaries and peritoneum. The effect of endometriosis on obstetric outcomes is still conflicting. The prevalence of endometriosis in women of reproductive age is about 6% to 10% [12]. Endometriosis causes two main symptoms, pain and infertility. It is well accepted that endometrium of women with endometriosis is abnormal. And, these abnormal endometrium may be the causes of the decidual impairment and abnormal placentation during pregnancy [34]. So, these processes may be have bad impacts on pregnancy outcomes. Also, the expression of inflammatory cytokines are the same factors implicated in the endometrium of endometriosis and trophoblast of preterm birth. However, studies on the relation between preterm birth and endometriosis are conflicting opinions, with some studies reporting an significant increased risk of preterm birth [567891011] and others showing some or not [12].
The objective of our study was to examine whether primiparous singleton pregnant women with endometriosis are associated with increased risk of preterm birth.
We developed a search strategy to use MeSH (Medical Subject Heading) terms and free key words and text words related to “endometriosis,” “pregnancy,” “adverse pregnancy outcomes,” “preterm birth,” and “cesarean section.” We searched these terms from PubMed, Medline, KERIS (Korea Education and Research Information Service), Scopus, and Google Scholar from March 1994 through February 2016 database without language restrictions. The inclusion criteria were all published prospective, retrospective cohort study, large population based prospective cohort study, retrospective secondary analysis, and multicentric, observational and cohort study on the relations between endometriosis and pregnancy prognosis, while publications in abstract form alone were excluded. Data abstraction was completed by two independent investigators. Each independently abstracted data from each study and analyzed data separately.
The endometriosis group with pelvic endometriosis confirmed histologically and visually at the surgical procedure before pregnancy or after section. The unexposed group included women who did not have a previous surgical or clinical diagnosis of endometriosis, and who did not have any ultrasonographic sign of endometriosis. Women with multiparous women, multiple pregnancies, malignancies, autoimmune disease, and cardiovascular diseases were also excluded. Preterm birth defined as live birth above 20 weeks before 37 completed weeks.
Differences were reviewed, and further resolved by common review of the entire data set. Data abstracted included number of study patients, number of patients in endometriosis groups and control groups, and adverse pregnancy outcomes. Six studies that did not stratify data, composite data were extracted. When possible, authors of included trials were contacted for missing data. The risk of bias in each included study was assessed by using the criteria outlined in the Risk of Bias Assessment Tool for Non-randomized Studies. Seven domains related to risk of bias were assessed in each included trial since there is evidence that these issues are associated with biased estimates of treatment effect: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome date, 6) selective reporting, and 7) other bias. Review authors' judgments were categorized as low, high, or unclear risk of bias.
Meta-analyses were performed with random effects models according to the heterogeneity of studies and we were trying to estimate the mean of a distribution of true effects. The completed analyses were then compared, and any difference was resolved with review of entire data set and independent analysis. Statistical heterogeneity between studies was assessed using Cochrane Q statistics and Higgins I2 statistics (P-value of the Cochrane Q statistic and Higgins I2 statistic <0.1) (Q (5)=11.645, P=0.040).
All effect sizes were calculated through Comprehensive Meta-Analysis ver. 2.0 (Biostat, Englewood, NJ, USA). Meta-analyses were estimated with odds ratios and 95% confidence intervals using random effect analysis according to heterogeneity of studies. P-value less than 0.05 was considered statistically significant. The meta-analysis was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement. This study had no funding source.
Seventeen trials on adverse obstetric outcomes in women with endometriosis were identified. Seven studies evaluating the adverse effect of endometriosis on pregnancy outcome by comparing women with endometriosis with women without endometriosis. Six trials that met inclusion criteria for this meta-analysis were analyzed. No similar meta-analysis was found. Fig. 1 shows the flow diagram of information through the different phases of the review [5678910]. Table 1 shows the summary of characteristics of enrolled studies included meta-analyses [5678910].
Fig. 2 shows funnel plot for assessing publication bias for the risk of endometriosis and preterm birth; it looked apparent that the funnel plot in Fig. 2 was symmetric. We performed additional tests to check if there was a bias because of small studies. With Begg and Mazumdar's rank correlation approach, Kendall's tau is 0.2 which was not statistically significant (P>0.05). These results indicated that the bias was unlikely in this data. Furthermore, we scrutinized what it would be like if the data was perfectly symmetric with Duval and Tweedie's trim and fill approach. Six virtual studies were added to make the data symmetric. The adjusted estimates was 1.473 which was not that statistically deviant from the observed value 1.355, which suggested that the given estimates were reliable and constant regardless of the adjustment (Fig. 2).
Endometriosis is a common and painful disease affecting women of reproductive age. While the underlying pathophysiology is still largely unknown, much advancement has been made in understanding the progression of the disease. The results of diverse studies on the connection between endometriosis and pregnancy outcomes are different, with some results publishing an increased complications and others showing some or no relations [1314]. Preterm birth (<37 weeks of gestation) is the major cause of perinatal morbidity and mortality globally, and affects 5% to 15% of all pregnancies, and its prevention is a significant healthcare priority. Preterm birth is one of the great obstetrical syndromes and is occurred by multiple causes [1516]. The biochemical materials associated in parturition, both at preterm and term, include the increased expression of inflammatory cytokines such as interleukin 6, interleukin 1β, and tumor necrosis factor α. These local and systemic inflammatory cytokines are the same factors implicated in the endometrium of endometriosis and trophoblast of preterm birth [17]. Also, the levels of prostaglandin E2, cyclooxygenase 2 and various cytokines are highly elevated in endometriotic tissue relative to normal endometrium. In preterm birth, the hypermethylation of progesterone receptor (PR)-B promotor and PR isoform A are more expressed than PR-B as well as in endometriosis [18]. These meta-analyses of pooled datas of four studies shows that pregnant women with endometriosis are significantly associated with increased risk of preterm birth.
Many studies have been included women with clinical endometriosis in whom a definitive diagnosis was unobtainable, and women who were pregnant through assisted reproductive technologies (ARTs), which in itself is a risk factor for preterm birth. In Mekaru et al. [5], they excluded conceived women through in vitro fertilization-embryo transfer and without an accurate diagnosis of endometriosis on laparoscopy. We must be careful regarding the effects of infertility itself on pregnancy adverse outcomes like as preterm birth, cesarean birth, and preeclampsia [19]. In addition, removal and simultaneous additional treatment of endometriosis may have improved pregnancy outcomes [2021]. The limitation of Aris [6] is that they did not take into account the order of endometriosis diagnosis or reproduction procedures (natural, ART). The strengths of Stephansson et al. [7] publication are large population and the preterm birth risk comparison between endometriosis women with ART and without ART. They found an increased risk of preterm birth among women aith endometriosis, and relative risk of preterm birth remained unchanged when stratified for the use of ART. The risk of preterm birth associated with endometriosis among with ART was 1.24, and among women without ART 1.37 [5]. In the study of Conti et al. [8], all patients had confirmed pathologic diagnosis of endometriosis before first pregnancy by surgical removal of the lesions, resulting ovarian (35%), mixed ovarian and peritoneal (25%), mixed ovarian and deep (21%) and deep lesions (19%). Data on the space in time between surgery and first pregnancy, as well as the additional treatment, were not gathered. The merits of Lin et al. [9], all women with endometriosis were diagnosed during surgery and confirmed histologically. The research of Stern et al. [10], targeted many women. In general, the rate of obstetrics complications was much higher during first pregnancy, while theses conditions did not occur in subsequent pregnancy of multiparous women. This fact suggest a protective role of pregnancy in endometriosis towards second pregnancies, perhaps through immunological and hormonal modification [22]. Also ART and subsequent multiple pregnancies associated with endometriosis themselves are risk factors for preterm birth. If those factors are not adjusted, the association between endometriosis and preterm birth would remain to be unclear. However, in this analysis, ART adjustment was not performed. It is the limitation of this study.
More subspecialized classification (removal and simultaneous additional treatment of endometriosis, order of endometriosis diagnosis, and availability and types of ART, primiparous versus multiparous, time interval between surgery and first pregnancy) is necessary to clarify the effects of endometriosis on preterm birth.
Our study demonstrate women with endometriosis have an increased risks of preterm birth at first singleton pregnancy. There needs to be the increased international effort to understand the etiology of endometriosis, explore preventative methods of preterm birth, and personalize treatment options.
Figures and Tables
Fig. 1
Flow of information through different phases of review. KERIS, Korea Education and Research Information Service.
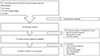
Table 1
Summary of characteristics of enrolled studies included meta-analyses

| Study (year) | Study design | Site (year) | Sample size (En+/En−) | Adjusted RR/OR (95% CI) |
|---|---|---|---|---|
| Mekaru et al. (2014) [5] | Retrospective study | Japan (1995–2011) | 108 (49/59) | 0.89 (0.18–4.24) |
| Aris (2014) [6] | cohort study | Canada (1997–2008) | 31,068 (784/30,284) | 1.15 (0.911–1.45) |
| Stephansson et al. (2009) [7] | Population-based prospective cohort | Sweden (1992–2006) | 13,090 (8,922/4,168) | 1.33 (1.23–1.44) |
| Conti et al. (2014) [8] | Multicentric, observational and cohort study | Italy (2010–2013) | 1,550 (219/1,331) | 2.24 (1.46–3.44) |
| Lin et al. (2015) [9] | Retrospective cohort study | China (1995–2013) | 498 (249/249) | 2.42 (1.05–5.57) |
| Stern et al. (2015) [10] | Retrospecive cohort study | USA (2004–2008) | 4,098 (590/3,508) | 1.66 (1.24–1.93) |
Table 2
The overall result of meta-analyses using random effects model

References
1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004; 364:1789–1799.
2. Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011; 96:360–365.
3. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011; 17:327–346.
4. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17:637–653.
5. Mekaru K, Masamoto H, Sugiyama H, Asato K, Heshiki C, Kinjyo T, et al. Endometriosis and pregnancy outcome: are pregnancies complicated by endometriosis a high-risk group? Eur J Obstet Gynecol Reprod Biol. 2014; 172:36–39.
6. Aris A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: impact of endometriosis. Gynecol Endocrinol. 2014; 30:34–37.
7. Stephansson O, Kieler H, Granath F, Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod. 2009; 24:2341–2347.
8. Conti N, Cevenini G, Vannuccini S, Orlandini C, Valensise H, Gervasi MT, et al. Women with endometriosis at first pregnancy have an increased risk of adverse obstetric outcome. J Matern Fetal Neonatal Med. 2015; 28:1795–1798.
9. Lin H, Leng JH, Liu JT, Lang JH. Obstetric outcomes in Chinese women with endometriosis: a retrospective cohort study. Chin Med J (Engl). 2015; 128:455–458.
10. Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015; 103:1438–1445.
11. Fernando S, Breheny S, Jaques AM, Halliday JL, Baker G, Healy D. Preterm birth, ovarian endometriomata, and assisted reproduction technologies. Fertil Steril. 2009; 91:325–330.
12. Omland AK, Abyholm T, Fedorcsak P, Ertzeid G, Oldereid NB, Bjercke S, et al. Pregnancy outcome after IVF and ICSI in unexplained, endometriosis-associated and tubal factor infertility. Hum Reprod. 2005; 20:722–727.
13. Vercellini P, Parazzini F, Pietropaolo G, Cipriani S, Frattaruolo MP, Fedele L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: a retrospective cohort study. BJOG. 2012; 119:1538–1543.
14. Kortelahti M, Anttila MA, Hippelainen MI, Heinonen ST. Obstetric outcome in women with endometriosis--a matched case-control study. Gynecol Obstet Invest. 2003; 56:207–212.
15. Benaglia L, Bermejo A, Somigliana E, Scarduelli C, Ragni G, Fedele L, et al. Pregnancy outcome in women with endometriomas achieving pregnancy through IVF. Hum Reprod. 2012; 27:1663–1667.
16. Brosens I, Brosens JJ, Fusi L, Al-Sabbagh M, Kuroda K, Benagiano G. Risks of adverse pregnancy outcome in endometriosis. Fertil Steril. 2012; 98:30–35.
17. Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013; 19:406–418.
18. Zanatta A, Pereira RM, Rocha AM, Cogliati B, Baracat EC, Taylor HS, et al. The relationship among HOXA10, estrogen receptor α, progesterone receptor, and progesterone receptor B proteins in rectosigmoid endometriosis: a Tissue Microarray Study. Reprod Sci. 2015; 22:31–37.
19. Leone Roberti Maggiore U, Ferrero S, Mangili G, Bergamini A, Inversetti A, Giorgione V, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update. 2016; 22:70–103.
20. Hayashi M, Nakai A, Satoh S, Matsuda Y. Adverse obstetric and perinatal outcomes of singleton pregnancies may be related to maternal factors associated with infertility rather than the type of assisted reproductive technology procedure used. Fertil Steril. 2012; 98:922–928.
21. Aznaurova YB, Zhumataev MB, Roberts TK, Aliper AM, Zhavoronkov AA. Molecular aspects of development and regulation of endometriosis. Reprod Biol Endocrinol. 2014; 12:50.
22. Vigano P, Corti L, Berlanda N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil Steril. 2015; 104:802–812.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download