Abstract
This regulatory post-marketing surveillance study aimed to evaluate the therapeutic efficacy and safety of drospirenone (DRSP) 2 mg/estradiol (E2) 1 mg tablet in Korean postmenopausal women. A total of 4,149 patients were enrolled and the study was conducted at 207 clinical research centers. The patients' source data was collected between November 2006 and November 2012. More than 85% of patients experienced improvement of menopausal symptoms. The most frequently reported adverse events were vaginal bleeding and breast pain; most of the women suffering from these symptoms fully recovered. The incidence of adverse event was higher in patients of younger age (20 to 39 years), in patients with concomitant diseases, previous hormone replacement therapy in medical history, those treated with DRSP 2 mg/E2 1 mg for shorter duration (3 years or less) and in patients using concomitant medication. In conclusion, the results from this large post-marketing surveillance study confirm the efficacy and safety of DRSP 2 mg/E2 1 mg tablet in Korean postmenopausal women.
Menopausal hormone therapy is the most effective treatment for menopausal symptoms of any severity for women under the age of 60 or for women within 10 years of menopause [12]. Drospirenone (DRSP) in combination with 17 beta estradiol (E2) is currently used as hormone replacement therapy for postmenopausal women.
DRSP is a novel progestogen with aldosterone receptor antagonistic properties. Furthermore, DRSP differs from most synthetic progestogens as it demonstrates a similar pharmacological profile to endogenous progestogens [345]. Consequently, DRSP is devoid of any estrogenic, glucocorticoid or anti-glucocorticoid activity but exhibits anti-aldosterone and anti-androgenic properties. Importantly, the anti-aldosterone properties of DRSP show the potential for promising results on weight gain and elevated blood pressure. These positive effects may be attributed to counterbalancing estrogen related water retention when used in a hormone therapy preparation [678].
The safety and efficacy of the DRSP 2 mg/E2 1 mg for the treatment of climacteric symptoms and for the prevention of postmenopausal osteoporosis have been confirmed in several studies [9]. This study aimed to evaluate the efficacy and safety of DRSP 2 mg/E2 1 mg tablet in Korean postmenopausal women.
The source data used in this report were collected between November 2006 and November 2012 from the case report forms of 4,149 patients who had taken DRSP 2 mg/17 β-E2 1 mg (Angeliq, Bayer Healthcare, Berlin, Germany) from 207 different centers. 4,078 patients were evaluated for safety, 2,964 patients were evaluated for efficacy. The efficacy was assessed in subjects who continue to take the study drug up to 3 cycles. In addition, the efficacy data from subjects who discontinue taking the study drug within 3 cycles due to safety reason was also included in the efficacy assessment.
The severity of symptoms were categorized following patients' subjective expression as well as clinicians' opinions and the change in severity of postmenopausal symptoms such as hot flushes, sweating attacks, vaginal dryness, nervousness, depressive moods, sleep disturbance, urinary frequency and nocturia were recorded.
Any available data including underlying diseases in medical history, allergic history, previous use of other female hormonal medicine, use of concomitant drugs, duration of DRSP 2 mg/E2 1 mg tablet treatment and potential factors that may be related to the reported adverse events for each case with reported adverse event were thoroughly reviewed.
In cases of reported unexpected adverse events and serious adverse events (SAEs), any occurrence was carefully investigated and, the presence of specific trend was investigated. An adverse event can be defined as any undesirable experience associated with the use of a medical product in a patient. The event can be regarded as serious and should be reported to US Food and Drug Administration when the patient outcome is: death, life threatening, hospitalization, disability or permanent damage, congenital anomaly/birth defect, required intervention to prevent permanent impairment or damage, and other serious important medical events [10].
Efficacy analysis was performed using Wilcoxon signed-rank test to show proportion of severity level (none, mild, moderate, and severe) of menopausal symptoms at the start of treatment and the end of treatment. The severity change between the start and the end of treatment was also evaluated.
Adverse events have been summarized using the World Health Organization Adverse Reactions Terminology version 092 coding system. To assess the potential factors related to the occurrence of adverse events, logistic regression analysis was performed to consider various variables simultaneously.
A total of 4,149 patients were enrolled during the study period. Of these 4,149 patients, 44 patients received the drug before the contract and 27 patients were double enrolled and therefore excluded from the safety analysis. 4,078 patients were evaluated for safety. Further 426 patients who were treated less than 3 cycles and 688 patients whose efficacy was not assessable were excluded from the efficacy analysis so that a total of 2,964 patients were evaluated for efficacy.
The mean age of subjects at study start was 52.53±5.41 years old, and the most dominant age group was ‘50 to 59 years old’ with 2,581 patients (62.3%). The mean time since menopause was 4±3.97 years; time since menopause were 3 years and under in 1,982 patients (50.7%), over 3 years in 1,926 patients (49.3%). 2,140 patients (52.5%) experienced hormone therapy before treatment with DRSP 2 mg/E2 1 mg tablet (Table 1).
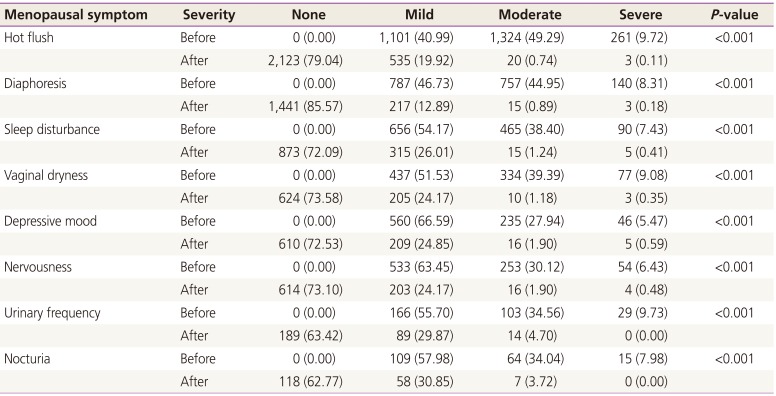
An efficacy evaluation was performed to assess the change in severity of symptoms at the start of treatment and the end of the treatment. Overall 86% of subjects, menopausal symptoms disappeared at the end of treatment (Table 2). Significant improvement of menopausal symptoms was reported; 97.8% of subjects with hot flush, 93.4% of subjects with diaphoresis, 90.3% of subjects with sleep disturbed, 93.4% of subjects with vaginal dryness, 88.0% of subjects with nervousness, 86.2% of subjects with depression, 85.6% of subjects with micturition frequency, and 81.9% of subjects with nocturia.
Five hundred seventy-five adverse events in 486 patients (11.9%) were reported during the study. The most frequently reported adverse event was vaginal bleeding (8.1%) and breast pain (4.0%) and followed by abdominal pain (0.3%), weight increase, hot flush (0.2%, each), vaginal dryness, and pruritus (0.1%, each).
Twenty-two SAEs occurred in 18 patients (0.4%) during the study. All cases except 1 were considered serious due to medical importance. The most commonly reported SAEs was vaginal bleeding. Eight cases were mild and five cases were moderate among 13 cases, there was no action taken to 11 cases. Regarding to the causal relationship between the event and treatment with DRSP 2 mg/E2 1 mg tablet, investigators evaluated that 1 case was ‘probably not related’, 5 cases were ‘possibly related’, 1 case was ‘certainly related’, and 6 cases were ‘probably related’. Except 2 cases reported ‘not recovered’ 11 cases of ‘vaginal bleeding’ were all recovered. Breast pain was reported in 6 cases as SAEs. Two cases were mild, 3 cases were moderate and 1 case was severe. There was no action taken to all 6 cases, and investigators evaluated that 1 case was ‘probably not related’, 3 cases were ‘possibly related’ and 2 cases were ‘certainly related’ as causal relationship with DRSP 2 mg/E2 1 mg tablet treatment. Six cases of breast pain were all recovered. In addition, hot flush, abdominal pain, and breast cancer were reported as SAEs. Hot flush was reported as ‘severe’, ‘no action taken’, ‘recovered with sequelae’ and investigator evaluated the event ‘certainly related’ with DRSP 2 mg/E2 1 mg tablet treatment. Abdominal pain was reported as ‘severe’, ‘no action taken’, ‘not recovered’, and ‘certainly related’ with treatment with DRSP 2 mg/E2 1 mg tablet. No information on the stage, action taken or outcome was available for the SAE case with breast cancer. Causal relationship with DRSP 2 mg/E2 1 mg tablet treatment was reported as ‘Unknown’.
Nine unexpected adverse events occurred in 9 patients (0.2%) which were lactation nonpuerperal, mammogram abnormal, gynecological-related pain, myalgia, body aching, fatigue, sensation of warmth, obesity central, and neck stiffness.
In the evaluation of factors affecting safety and the incidence of adverse events by logistic regression with multiple variables, the incidence of adverse events was higher in patients with lower age (odds ratio [OR], 0.98; 95% confidence interval [CI], 0.96 to 1.00; P=0.0443), short administration cycle (OR, 0.84; 95% CI, 0.80 to 0.87; P<0.0001), concomitant disease (OR, 1.62; 95% CI, 1.25 to 2.09; P=0.0003), previous hormone replacement therapy (OR, 1.93; 95% CI, 1.53 to 2.44; P<0.0001), concomitant medications than the patient who was not with(OR, 2.08; 95% CI, 1.61 to 2.68; P<0.0001).
The efficacy and safety of DRSP 2 mg/E2 1 mg in Korean postmenopausal women were evaluated in this multicenter, postmarketing surveillance study. The results of this study affirmed that the DRSP 2 mg/E2 1 mg was effective for the relief of vasomotor symptoms, sleep disturbance, vaginal dryness, nervousness and depression, and urinary symptoms.
The improvement rate for most symptoms proved to be highly positive as more than 90% of the women showed improvement in hot flashes, diaphoresis, sleep disturbance and vaginal discharge. Additionally more than 80% showed improvement in depression, nervousness, micturition frequency and nocturia.
These findings are consistent with another study that reported DRSP 2 mg/E2 1 mg to be significantly more effective in relieving Korean postmenopausal symptoms compared to placebo [11]. The study reported decreased hot flashes by 84.4% in the DRSP 2 mg/E2 1 mg group in comparison to 48.1% in the placebo group. In fact, the DRSP 2 mg/E2 1 mg group proved to be significantly more effective in relieving symptoms compared to the placebo group including hot flashes of all intensity, incidence of depression, nervousness, and vaginal dryness. The DRSP 2 mg/E2 1 mg group also displayed an 80% decrease in sweating episodes and a 49% decrease in insomnia.
A study that investigated different dosing regimen of 1 mg E2 with 1, 2 or 3 mg of DRSP reported a decrease in the number of hot flashes in all subjects by the 5th weeks of the study. It is noteworthy that the decrease was greater in women with moderate to severe hot flashes than the women with mild ones [12]. In comparison to the placebo group, positive results showing greater decrease in hot flashes could be observed in the active treatment groups from the 3rd to the 16th week of the study. Also, a decrease in the frequency and the severity of hot flashes within 2 weeks with no difference in the doses of DRSP has been reported [913].
In this study, the most common adverse event was vaginal bleeding at 8% followed by breast pain at 4%. Younger subjects and subjects with a shorter administration cycle experienced a higher rate of adverse events. These finding on higher incidence of vaginal bleeding in younger subjects or shorter treatment were consistent with previous reports and confirms that vaginal bleeding seems to be the most common adverse event which improves over time [9111213].
The strength of this study is as follows: this large prospective study involving 4,149 Korean women affirms the efficacy and safety of DRSP 2 mg/E2 1 mg as postmenopausal hormone therapy. The type and incidence of adverse events reported in this study were consistent with previous studies. It must also be noted that the severity of most adverse events including SAEs were mild to moderate and there were no fatal event. In case of SAEs, although investigators perceived these as serious with medical importance at first, most of them recovered well without further treatment. However the raw data didn't include the reasons for severity evaluation of SAE, so this can be considered limitation of this study.
This study has several important limitations. First, the trial was incomplete in that the facilities lost track of some patients. So, raw data has insufficient information in some cases. Since the information obtained from postmarketing surveillance is observational in nature and adverse events were measured using a questionnaire handed out, there is a high likelihood that occurrence of mild adverse events might have been underestimated.
References
1. Shifren JL, Gass ML. NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014; 21:1038–1062. PMID: 25225714.

2. de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013; 16:316–337. PMID: 23672656.

3. Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995; 761:311–335. PMID: 7625729.

4. Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential. Contraception. 1996; 54:243–251. PMID: 8922878.

5. Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000; 62:29–38. PMID: 11024226.

6. Losert W, Casals-Stenzel J, Buse M. Progestogens with antimineralocorticoid activity. Arzneimittelforschung. 1985; 35:459–471. PMID: 4039568.
7. Pollow K, Juchem M, Elger W, Jacobi N, Hoffmann G, Mobus V. Dihydrospirorenone (ZK30595): a novel synthetic progestagen: characterization of binding to different receptor proteins. Contraception. 1992; 46:561–574. PMID: 1493716.
8. Rubig A. Drospirenone: a new cardiovascular-active progestin with antialdosterone and antiandrogenic properties. Climacteric. 2003; 6(Suppl 3):49–54. PMID: 15018248.
9. Archer DF, Thorneycroft IH, Foegh M, Hanes V, Glant MD, Bitterman P, et al. Long-term safety of drospirenone-estradiol for hormone therapy: a randomized, double-blind, multicenter trial. Menopause. 2005; 12:716–727. PMID: 16278615.

10. US Food and Drug Administration. What is a serious adverse event? [Internet]. Silver Spring (MD): US Food and Drug Administration;2016. cited 2017 Feb 10. Available from: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.
11. Lee BS, Kang BM, Yoon BK, Choi H, Park HM, Kim JG. Efficacy and tolerability of estradiol 1 mg and drospirenone 2 mg in postmenopausal Korean women: a double-blind, randomized, placebo-controlled, multicenter study. Maturitas. 2007; 57:361–369. PMID: 17467203.
12. Schurmann R, Holler T, Benda N. Estradiol and drospirenone for climacteric symptoms in postmenopausal women: a double-blind, randomized, placebo-controlled study of the safety and efficacy of three dose regimens. Climacteric. 2004; 7:189–196. PMID: 15497908.

13. Archer DF. Drospirenone and estradiol: a new option for the postmenopausal woman. Climacteric. 2007; 10(Suppl 1):3–10. PMID: 17364592.

Table 1
Patients' clinical characteristics
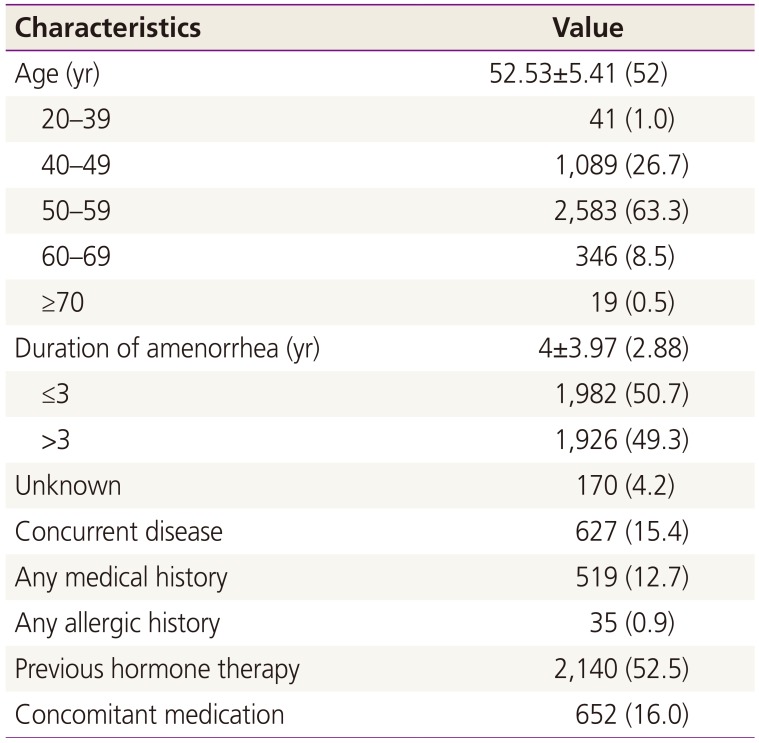
Table 2
Severity change of menopausal symptoms between the start of treatment and the end of medication




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download