Abstract
Objective
To evaluate thyroid function and hormonal profile in women with polycystic ovary syndrome (PCOS).
Methods
A case-control study was conducted at Saad Abualila Center, Khartoum, Sudan. The cases were women with confirmed PCOS based on Rotterdam criteria. The controls were infertile women with no evidence of PCOS. The socio-demographic characteristics and medical history were gathered using a questionnaire. Thyroid hormones (thyroid-stimulating hormone, free tri-iodothyronine, and free thyroxine), anti-thyroid peroxidase, and anti-thyroglobulin antibodies were measured.
Results
While there were no significant differences in the age and haemoglobin levels of the two studied groups (55 women in each arm), body mass index was significantly higher in women with PCOS. There were no significant differences in the levels of thyroid-stimulating hormone, luteinizing hormone, follicle stimulating hormone, luteinizing hormone/follicle stimulating hormone, anti-thyroid peroxidase, anti-thyroglobulin antibodies, cholesterol, triglycerides and low-density lipoprotein cholesterol between the cases and the controls. The mean±standard deviation of free tri-iodothyronine (3.50±0.2 vs. 3.38±0.3 pg/mL, P=0.040) and median (interquartile) high-density lipoprotein cholesterol (37.0 [34.0 to 42.0] vs. 35.80 [29.0 to 41.0] mg/dL, P=0.015) were significantly higher in PCOS patients compared with the control group. In linear regression, PCOS (0.151 pg/mL, P=0.023) and anti-thyroid peroxidase levels (-0.078 pg/mL, P=0.031) were significantly associated with free tri-iodothyronine.
Polycystic ovary syndrome (PCOS) is essentially a group of endocrine disorders that commonly affect women during reproductive age [1]. It is characterized by irregular, anovulatory menstrual cycles, features of hyperandrogenism, and polycystic ovaries [2]. Multiple endocrine derangements were described in patients with PCOS including abnormally high levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), FSH/LH, prolactin, testosterone, estradiol and insulin resistance [123]. The relatively high prevalence of insulin resistance among PCOS patients partly explains features of metabolic syndrome in the affected subjects, which include obesity, hypertension and dyslipidemia [4].
PCOS shares common features with hypothyroidism like abnormal menses, anovulatory cycles, obesity, dyslipidemia and psychological disturbances [5]. Coexistence of autoimmune thyroiditis and/or goiter with biochemical features of hypothyroidism is not uncommon among patients with PCOS [6]. In contrast, the results of some studies showed increased levels of tri-iodothyronine (T3) and anti-thyroid peroxidase (anti-TPO) antibody in PCOS patients [7], which confuse the common believe that hypothyroidism is a part of PCOS endocrinopathy. At least two reports document Graves' disease in PCOS patients attending endocrinology clinics for the purpose of follow-up [89]. Noteworthy, hyperthyroidism can also explain some features of PCOS like hypertension, abnormal menstrual cycles, psychological disturbances and low body mass index (BMI) in some of the affected patients [10]. Investigating thyroid function in women with PCOS is importance for the researchers as well as the treating physicians. There is no published data on thyroid functions and PCOS in Africa including Sudan. These contradictory views on the pattern of thyroid function in PCOS prompted us to conduct the present study, which aimed to evaluate thyroid function and hormonal profile in patients with PCOS. We have previously shown that PCOS was the main cause of female infertility in Sudan [1112].
This was a case-control study carried out at Saad Abualila infertility center (Khartoum, Sudan) during the period of January to December 2014. Saad Abualila Hospital is a tertiary semi-private hospital governed by the Faculty of Medicine, University of Khartoum. Cases were women with confirmed PCOS based on Rotterdam criteria [3], where at least two of the following criteria were fulfilled: oligomenorrhoea/anovulation (delayed menses >35 days or <8 spontaneous hemorrhagic episodes/yr), hyperandrogenism (clinical hirsutism using modified Ferriman-Gallwey score of ≥8 or biochemical) and morphology of polycystic ovaries on ultrasonography (12 or more follicles in each ovary measuring 2 to 9 mm in diameter, and/or increased ovarian volume >10 mL3).
Controls were infertile women with no evidence of PCOS presented to the same fertility clinic and had regular menstrual cycles (21 to 35 days), no evidence of hirsutism and no polycystic ovary morphology on ultrasonography.
Women with systemic disease (cardiovascular disease and diabetes mellitus), on medication for ≤6 months prior to the study (oral contraceptives, glucocorticoids, ovulation induction agents, and estrogenic or anti-androgenic) were excluded from the cases and the controls.
After signing an informed consent; the socio-demographic characteristics, medical and gynecological history were taken from each patient using a questionnaire. The detailed medical and gynecological history (menstrual pattern, fertility, hirsutism, acne, acanthosis nigricance, scalp hair loss or thinning) were taken from all patients included in the study. Then full general and pelvic examinations were performed.
Body weight (kg), height (cm), were measured in all women. BMI was calculated by dividing the weight (in kg) by the squared height (m2). Ultrasonography was performed with a 6.5 MHz frequency and vaginal transducer where presence or absence of ovarian cysts were conformed or excluded. If cysts were seen, then the volume as well as the number of small follicles were determined, in each ovary.
Then 5 mL of venous blood was taken from each respondent, allowed to clot, centrifuged, and stored at -20℃ using immunoassay analyzer (AIA 360, Tosoh, Tokyo, Japan), following the manufacturer's instructions, till the assay of the thyroid hormones (thyroid-stimulating hormone [TSH], free T3, and free thyroxine [T4]), prolactin, FSH, LH. Specific anti-TPO and anti-thyroglobulin (anti-TG) antibody profiles were analyzed using commercial ELISA (Euroimmun, Lübeck, Germany) kits as we described elsewhere [13].
Total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and triglycerides were measured using an enzymatic colorimetric method. A total sample size of 55 women in each arm of the study groups (cases and controls) was calculated using a formula for the difference in the mean of the proposed variables (TSH, free T3, free T4, anti-TPO, and anti-TG antibodies) that would provide 80% power to detect a 5% difference at α=0.05 and which assumed that 10% of women would not respond or have incomplete data.
Data were entered into a computer using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Continuous data were compared between the two groups (cases and controls) using t-test and Mann-Whitney U-test when the data were normally and abnormally distributed, respectively. Simple linear regression models were performed where, TSH, free T3, free T4 were continuous dependent variables and age, BMI index and PCOS were the independent predictors of interest. A P-value of <0.05 was considered significant.
The study received ethical clearance from the research board at the Faculty of Medicine and Health Sciences, Alneelain University, Khartoum, Sudan.
While there were no significant differences in the age and haemoglobin levels of the two studied groups), BMI was significantly higher in women with PCOS (Table 1). There were no significant differences in the levels of TSH, LH, FSH, LH/FSH, testosterone, prolactin, anti-TPO, anti-TG antibodies, cholesterol, triglycerides and LDL between the cases and the controls (Table 2).
The mean±standard deviation of free T3 (3.50±0.2 vs. 3.38±0.3 pg/mL, P=0.040) and median (interquartile) HDL (37.0 [34.0–42.0] vs. 35.80 [29.0–41.0] mg/dL, P=0.015) were significantly higher in PCOS patients compared with the control group (Table 2, Fig. 1). In linear regression analysis, presence of PCOS (0.151 pg/mL, P=0.023) and anti-TPO levels (-0.078 pg/mL, P=0.031) were significantly associated with free T3 (Table 3).
According to the present results, free T3 was significantly higher among PCOS patients compared with the control group and inversely associated with anti-TPO levels. The finding of higher free T3 among PCOS patients goes the few reports that documented hyperthyroidism in cases with PCOS [789], but disagree with significant number of other studies that repeatedly demonstrated hypothyroidism in PCOS patients [614151617].
A prospective multicenter study demonstrated a threefold higher prevalence of autoimmune thyroiditis in patients with PCOS. Measurements of thyroid parameters revealed higher TSH and anti-TPO antibody, but lower T3 and T4 and in PCOS patients compared with the control group. Further assessment of hormonal status showed significantly increased FSH, LH, FSH/LH, testosterone and estradiol in PCOS patients; however, progesterone levels were comparable between the test and the control groups [6]. Based on evaluation of the homeostatic model assessment insulin resistance index, the degree of insulin resistance was higher among PCOS patients; however, the study did not assess other indicators of glycemic control or lipids profile among the affected patient [6]. In a Chinese study, the functions of the pituitary, ovary, adrenal cortex and thyroid gland were assessed in healthy controls and two subsets of PCOS patients with LH/FSH ≥ or <3 respectively [14]. Following TRH administration, exaggerated responses of TSH and prolactin were noted in the two PCOS groups; however, there was blunted free T4 response in those with LH/FSH ≥3 compared with the controls. The study suggested increased risk of subclinical hypothyroidism in PCOS patients with LH/FSH ≥3. In a comparable Italian study, administration of antidopaminergic drug preferentially increased basal secretion prolactin and TSH in PCOS patients compared to normal controls, while levels of LH and FSH remained unchanged [15]. The study suggested depressed hypothalamic dopaminergic activity in PCOS patients. The abnormal regulation of prolactin and thyroid function was also demonstrated in another two Turkish [16] and Russian [17] studies.
High prevalence of autoimmune thyroiditis and goiter were demonstrated among PCOS patients studied by Sinha et al. [7]. Interestingly, Sinha et al. [7], results also showed increased levels of T3 anti-TPO antibody among PCOS patients, which was similar to our present findings. High thyroid hormones secondary to Graves' disease levels were also demonstrated in six PCOS ladies attending an endocrinology clinic in north India [8] and another Asian lady visited the Hospital of Gyeongsang National University, South Korea [9]. The association of an infrequent pathology like Graves' disease with a fairly common condition like PCOS is unlikely accidental and should motivate researchers to investigate more in this field.
Noteworthy, the BMI of the PCOS patients we studied was higher than the controls. In addition, serum lipids were apparently higher in PCOS patient than healthy control group; however, only HDL achieved statistical significance. It has been hypothesized that obese PCOS patients are at higher risk of insulin resistance and hyperandrogenism than normal-weight PCOS women [18]. A review 30 eligible studies aiming to evaluate the effects of high BMI on the metabolic features of PCOS showed that; in comparison with non-obese, obese women with PCOS had increased androgens, fasting glucose, fasting insulin, homeostatic model assessment insulin resistance index and worsened lipid profile [19]. In another review article, Cibula showed evidences that patients with PCOS are likely to have insulin sensitivity comparable with healthy subjects if both groups were meticulously matched for the possible confounding factors [20]. In the present study, there were no differences between PCOS patients and the control groups regarding the hormones known to predominate in PCOS, in addition to other sociodemographic characteristics. This well matching among the groups we studies may preclude presence of insulin resistance in our PCOS patients based on Cibula hypothesis and hence explains the comparable lipid profile in our study groups. However, another question remained for further investigations and researches; why TSH, LH, FSH, LH/FSH, testosterone, prolactin, anti-TPO and anti-TG antibodies were not significantly different in the PCOS and the control groups we studied?
Evaluation of iodine homeostasis is essential in studies assessing thyroid functions, a fact that makes absence of serum and/or urine iodine measurements a potential limitation of this study. However, significantly increased T3 in the PCOS patients compared with the control we studied exclude iodine insufficiency as a probable cause of thyroid over activity in the first group. Moreover, fasting insulin and glucose levels, if assessed among the studied groups, could have indicated the state of insulin sensitivity/resistance among PCOS patients we evaluated [21].
The present study demonstrated significantly higher T3 among PCOS patients compared with the healthy controls, which add evidence to the few reports that confirmed the association between PCOS and hyperthyroidism. Failure of the present data to show significant differences in TSH, LH, FSH, LH/FSH, testosterone, prolactin and thyroid antibodies between PCOS patients and the control group remains for further investigations by researchers in the field.
References
1. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013; 6:1–13. PMID: 24379699.

2. Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibanez L, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015; 4. 01. DOI: 10.1159/000375530. [Epub].

3. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81:19–25.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. PMID: 11368702.
5. Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013; 17:138–145. PMID: 23776867.

6. Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gartner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004; 150:363–369. PMID: 15012623.

7. Sinha U, Sinharay K, Saha S, Longkumer TA, Baul SN, Pal SK. Thyroid disorders in polycystic ovarian syndrome subjects: a tertiary hospital based cross-sectional study from Eastern India. Indian J Endocrinol Metab. 2013; 17:304–309. PMID: 23776908.

8. Nisar S, Shah PA, Kuchay MS, Bhat MA, Rashid A, Ahmed S, et al. Association of polycystic ovary syndrome and Graves' disease: is autoimmunity the link between the two diseases. Indian J Endocrinol Metab. 2012; 16:982–986. PMID: 23226647.
9. Jung JH, Hahm JR, Jung TS, Kim HJ, Kim HS, Kim S, et al. A 27-year-old woman diagnosed as polycystic ovary syndrome associated with Graves' disease. Intern Med. 2011; 50:2185–2189. PMID: 21963738.

10. Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal androgen excess and body mass index in polycystic ovary syndrome. J Clin Endocrinol Metab. 2015; 1. 07. DOI: 10.1210/jc.0000-9999. [Epub].

11. Elussein EA, Magid YM, Omer MM, Adam I. Clinical patterns and major causes of infertility among Sudanese couples. Trop Doct. 2008; 38:243–244. PMID: 18820200.

12. Ahmed M, Shareef O, Adam I, Rayis D. Maternal age and intracytoplasmic sperm injection outcome in infertile couples at Khartoum, Sudan. F1000Res. 2015; 4:1339. PMID: 27347370.

13. Elhaj ET, Adam I, Alim A, Elhassan EM, Lutfi MF. Thyroid function/antibodies in Sudanese patients with preeclampsia. Front Endocrinol (Lausanne). 2015; 6:87. PMID: 26124747.

14. Wu X, Zhang Z, Su Y. Functional states of pituitary-ovary, adrenal and thyroid axes in women with polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi. 1998; 33:153–156. PMID: 10682483.
15. Zironi C, Pantaleoni M, Zizzo G, Coletta F, Velardo A. Evaluation of dopaminergic activity of the hypothalamus in patients with polycystic ovarian syndrome. Minerva Ginecol. 1991; 43:443–447. PMID: 1685015.
16. Gulekli B, Turhan NO, Senoz S, Kukner S, Oral H, Gokmen O. Endocrinological, ultrasonographic and clinical findings in adolescent and adult polycystic ovary patients: a comparative study. Gynecol Endocrinol. 1993; 7:273–277. PMID: 8147237.
17. Tudose TI, Zelenetskaia VS, Kozlov GI, Pishchulin AA, Dobracheva AD. Lactotropic and thyrotropic functions of the hypophysis in polycystic ovary syndrome. Probl Endokrinol (Mosk). 1986; 32:3–7.
18. Lin JF, Li X, Zhu MW. Exploration of the classification of polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi. 2006; 41:684–688. PMID: 17199924.
19. Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013; 14:95–109. PMID: 23114091.

20. Cibula D. Is insulin resistance an essential component of PCOS? The influence of confounding factors. Hum Reprod. 2004; 19:757–759. PMID: 15033943.

21. Preethi BL, Jaisri G, Kumar KM, Sharma R. Assessment of insulin resistance in normoglycemic young adults. Fiziol Cheloveka. 2011; 37:118–125. PMID: 21466052.

Fig. 1
(A-C) Thyroid functions in cases and controls. TSH, thyroid-stimulating hormone; T3, tri-iodothyronine; T4, thyroxine.
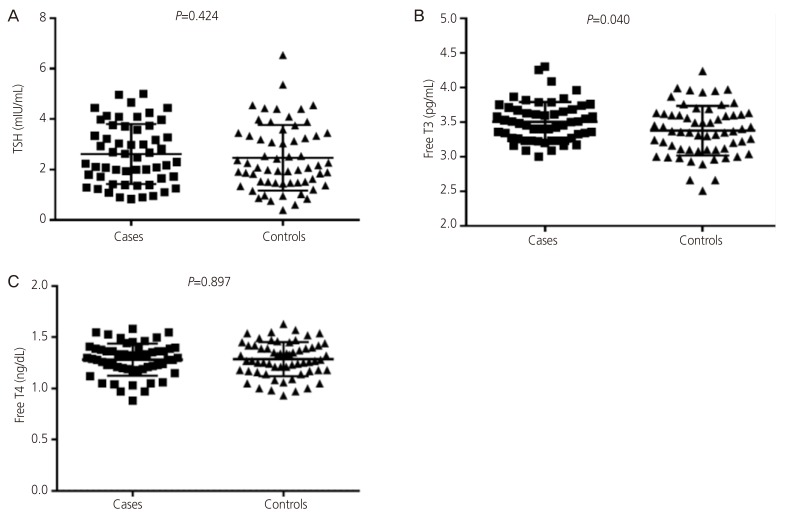
Table 1
Mean±standard deviation of the age, body mass index, and hemoglobin in the cases and controls

| Variable | Cases (n=55) | Controls (n=55) | P-value |
|---|---|---|---|
| Age (yr) | 27.3±5.2 | 27.1±4.8 | 0.840 |
| Body mass index (kg/m2) | 28.2±5.3 | 25.3±5.8 | 0.006 |
| Hemoglobin (g/dL) | 11.0±0.6 | 11.0±0.7 | 0.716 |
Table 2
Median (interquartile) of the hormones and lipid profile in women with polycystic ovary syndrome and controls
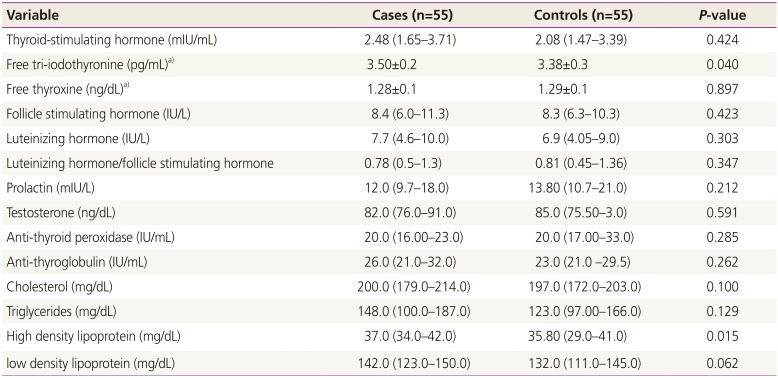
Table 3
Linear regression analysis of factors associated with TSH, free T3, and free T4 levels
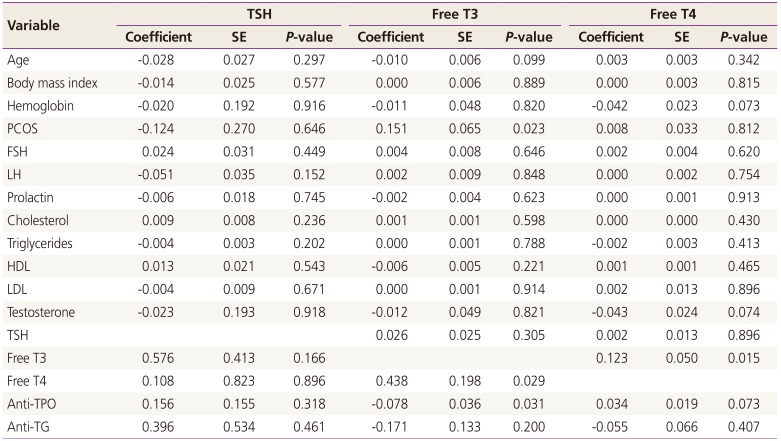
TSH, thyroid-stimulating hormone; T3, tri-iodothyronine; T4, thyroxine; SE, standard error; PCOS, polycystic ovary syndrome; FSH, follicle stimulating hormone; LH, luteinizing hormone; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TPO, thyroid peroxidase; TG, thyroglobulin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download