Abstract
Congenital mesoblastic nephroma is a rare renal tumor that is diagnosed during pregnancy and is associated with polyhydramnios, prematurity, and neonatal hypertension. Differential diagnoses include Wilms tumor, adrenal neuroblastoma, and other abdominal tumors. We report a case of congenital mesoblastic nephroma detected by prenatal ultrasonography as a large fetal renal mass with polyhydramnios at 32 weeks of gestation. Ultrasonography showed a 6×6-cm complex, solid, hyperechoic, round mass in the right kidney. At 35 weeks of gestation, the patient was admitted with preterm premature rupture of membranes and the baby was delivered vaginally. Postnatal ultrasonography and computed tomography showed a heterogeneous solid mass on the right kidney. At the end of the first week of life, a right nephrectomy was performed and subsequent pathological examination confirmed a cellular variant of congenital mesoblastic nephroma with a high mitotic count. Postoperative adjuvant chemotherapy was administered. The newborn was discharged in good condition.
Renal tumors are infrequently diagnosed in infants younger than 6 months of age [12], and neonatal renal tumors account for only 7% of all neonatal tumors [134]. Congenital mesoblastic nephroma (CMN) is a rare renal tumor that is frequently associated with polyhydramnios, premature birth, and neonatal hypertension [5]. Its differential diagnoses are important. While CMN is more common than Wilms tumor, it is difficult to distinguish CMN from Wilms tumor using ultrasonography (USG) [1678]. Treatment for CMN consists of nephrectomy followed by adjuvant chemotherapy if the pathological findings predict metastases or recurrence [2]. We report one case of a fetal renal mass detected by prenatal USG in a mother who became pregnant with an embryo generated by in vitro fertilization.
The mother was a 39-year-old primigravida who became pregnant after her 5th trial of in vitro fertilization. She had a history of arrhythmia (second-degree atrioventricular block and transient, complete atrioventricular block) that was treated with the insertion of a pacemaker. Results of an antenatal examination performed at a private clinic were normal, but those of a 50 g oral glucose tolerance test (OGTT) were above normal range (150 mg/dL); a 100 g OGTT was not performed. At 32 weeks 1 day of gestation, she visited the outpatient clinic at our tertiary center for prenatal care. USG showed mild polyhydramnios with an amniotic fluid index (AFI) of 24.8 cm, but fetal structural abnormalities were not observed at first USG. Because the estimated fetal weight was between the 75th and 90th percentiles and polyhydramnios was present as complication, gestational diabetes was suspected and a 100 g OGTT was performed. The following results were obtained: 73 mg/dL (fasting glucose level), 167 mg/dL (1 hr), 162 mg/dL (2 hr), and 142 mg/dL (3 hr). She was subsequently treated for gestational diabetes. Follow-up USG at 35 weeks revealed a well-circumscribed, homogeneous mass in the right kidney, measuring 5.6×4.8 cm (Fig. 1). The AFI also increased compared with that on the previous USG (AFI 36.4 cm). This antenatally detected fetal abdominal mass was considered to be a neuroblastoma or Wilms tumor.
The mother was admitted at a gestational age of 35 weeks 5 days due to preterm premature rupture of membranes and spontaneous labor was started and a pediatrician took part in delivery moment. A male baby weighing 2.55 kg was born vaginally and had an Apgar score of 9 and 10 at 1 and 5 minutes, respectively. Two days after birth, computed tomography (CT) revealed a heterogeneous renal mass measuring 5.3×5.2×4.2 cm and involving the mid- and lower-pole of the right kidney. Preoperative CT suggested the possibility of mesoblastic nephroma or a congenital Wilms tumor.
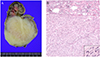
Three days after birth, the neonate underwent a nephrectomy by a pediatric surgeon. The renal mass (6.5×5.0×5.0 cm) originated from the lower pole of the right kidney and was confined to the kidney (Fig. 2). Inspection of the cut section revealed that the mass was round, solid, and well circumscribed, and the cut surface was yellowish white with a whirling pattern. Microscopic examination showed that the mass was composed of intersecting bundles of spindle cells with frequent mitoses (>20/10 high power field) (Fig. 2B), and was diagnosed as a cellular variant of mesoblastic nephroma. The baby was refered to pediatrics and has undergone six cycles of chemotherapy with vincristine (0.1 to 0.15 mg), dactinomycin (0.02 to 0.04 mg), and cyclophosphamide (35 to 50 mg). Baby experienced mild nausea, vomit, and fever. But, there were no any other serious side effects. Currently, the baby is 8 months old, with no signs of tumor recurrence on CT.
Despite the widespread use of USG to evaluate the fetus during pregnancy, only 15% of renal masses are identified prenatally [4]. Neonatal renal tumors account for only 7% of all neonatal tumors [134]. CMN is the most common neonatal renal tumor and is more prevalent than Wilms tumor in infants younger than 3 months of age [3]. CMN is more likely to occur in males and in the right kidney [39]. CMN is frequently associated with polyhydramnios and premature birth. In our case, polyhydramnios was detected at a gestational age of 32 weeks and aggravated rapidly thereafter. Mesoblastic nephroma usually manifests in the third trimester, with a rapid and unexplained increase in tumor size. Occasionally, these tumors may be large enough to cause abdominal dystocia, necessitating a cesarean section [10]. Polyhydramnios is observed in almost 70% of CMN cases [3]. The mechanism of polyhydramnios is not clear, but it may be due to intestinal obstruction by the renal mass and polyuria due to increased renal perfusion [111]. Polyhydramnios may also induce preterm delivery. In a rare case, CMN was reported to be associated with oligohydramnios [12]. In the oligohydramnios case, the baby with a renal mass diagnosed as CMN showed signs of anuria, hypotension, hyperkalemia, and disseminated intravascular coagulopathy after birth. As expected, babies with oligohydramnios have a poorer prognosis than those with polyhydramnios [12].
Overall, neonatal renal tumors have a good prognosis [3]. If a renal tumor is found, an accurate differential diagnosis is essential for proper treatment. Both mesoblastic nephroma and Wilms tumor are relatively common. In fact, mesoblastic nephroma is the most common tumor in infants younger than 3 to 6 months who have clinical symptoms of hypertension and hematuria or hyperreninism. However, it can be diagnosed prenatally by the presence of a fetal renal mass and polyhydramnios. Wilms tumor is also a common renal tumor in infants with clinical characteristics and USG features similar to those of mesoblastic nephroma. Therefore, differentiating between mesoblastic nephroma and Wilms tumor using antenatal USG is difficult. In our case, we considered that the probability of Wilms tumor was higher than that of mesoblastic nephroma, but the final pathologic analysis revealed CMN. A previous study indicated that a considerable portion of masses that are initially thought to be a Wilms tumor was ultimately diagnosed as CMN in the postnatal period, emphasizing the importance of considering CMN in the diagnosis of a renal mass detected during the antenatal period [9]. Other differential diagnoses include an ossifying renal tumor, neuroblastoma, cystic nephroma, and prenatal adrenal mass. Very rarely, high-risk tumors such as a clear cell sarcoma of the kidney or a malignant rhabdoid tumor can occur [34].
Diagnosis of a renal tumor depends on the exclusion a mass arising from the liver or adrenal gland. On USG, congenital adrenal tumor is separated from the kidney with definite margin and the observation of asynchronous movement between the mass and the kidney during fetal breathing can help differential diagnosis [1].
Classic CMN appears as a hypoechogenic tumor with an echogenic rim or a monogeneous or heterogeneous solid mass with no discernible rim; therefore, differentiation from Wilms tumor or other renal tumors is not possible using USG. Fetal magnetic resonance imaging (MRI) can play an important role in the differential diagnosis of an abdominal mass because it has the advantage of clearly distinguishing the margin of the tumor from normal kidney and adrenal gland tissue [1]. In our case, fetal MRI was not performed, and CT after birth was performed. MRI or CT can also be used for the evaluation of metastases or recurrence after nephrectomy [113].
The prognosis of CMN is generally good. The 5-year survival and overall survival rates of infants are 94% and 96%, respectively. These rates also depend upon the histological findings. Mesoblastic nephroma has three variants: classic/leiomyomatous, cellular/atypical, and mixed variants.
The cellular variant has a 5-year survival rate of 85% and an overall survival rate of 90% [3]. Although CMN is a benign tumor and the majority of patients are treated by surgical resection (i.e., nephrectomy), recurrence and metastases to the brain, lung, and heart have been reported [13]. In our case, the pathological finding was mesoblastic nephroma with frequent mitosis, so adjuvant chemotherapy was performed to lower the risk of metastases or recurrence.
Figures and Tables
Fig. 1
Prenatal ultrasonography reveals a well-circumscribed, solid, hyperechoic mass in the right kidney, measuring 5.6×4.8 cm. (A) Transverse section. (B) Longitudinal section.
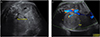
Fig. 2
Nephrectomy: cut sections and pathologic findings. (A) The mass originated from the lower pole of the right kidney. The cut surface is yellowish white with a whirling pattern. Lower, mass; upper, normal. (B) Hematoxylin and eosin stain, ×100. Microscopically, the mass is composed of intersecting bundles of spindle cells with frequent mitoses (inset).
References
1. Chen WY, Lin CN, Chao CS, Yan-Sheng Lin M, Mak CW, Chuang SS, et al. Prenatal diagnosis of congenital mesoblastic nephroma in mid-second trimester by sonography and magnetic resonance imaging. Prenat Diagn. 2003; 23:927–931.
2. Glick RD, Hicks MJ, Nuchtern JG, Wesson DE, Olutoye OO, Cass DL. Renal tumors in infants less than 6 months of age. J Pediatr Surg. 2004; 39:522–525.
3. Shapiro E. Upper urinary tract anomalies and perinatal renal tumors. Clin Perinatol. 2014; 41:679–694.
4. Powis M. Neonatal renal tumours. Early Hum Dev. 2010; 86:607–612.
5. Miller OF, Kolon TF. Hyperreninemia and congenital mesoblastic nephroma: case report and review of the literature. Urology. 2000; 55:775.
6. Ko SM, Kim MJ, Im YJ, Park KI, Lee MJ. Cellular mesoblastic nephroma with liver metastasis in a neonate: prenatal and postnatal diffusion-weighted MR imaging. Korean J Radiol. 2013; 14:361–365.
7. Garnier S, Maillet O, Haouy S, Saguintaah M, Serre I, Galifer RB, et al. Prenatal intrarenal neuroblastoma mimicking a mesoblastic nephroma: a case report. J Pediatr Surg. 2012; 47:e21–e23.
8. Rubenstein SC, Benacerraf BR, Retik AB, Mandell J. Fetal suprarenal masses: sonographic appearance and differential diagnosis. Ultrasound Obstet Gynecol. 1995; 5:164–167.
9. Wang ZP, Li K, Dong KR, Xiao XM, Zheng S. Congenital mesoblastic nephroma: clinical analysis of eight cases and a review of the literature. Oncol Lett. 2014; 8:2007–2011.
10. Woodward PJ, Sohaey R, Kennedy A, Koeller KK. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005; 25:215–242.
11. Shibahara H, Mitsuo M, Fujimoto K, Muranaka J, Sawai H, Bessho T, et al. Prenatal sonographic diagnosis of a fetal renal mesoblastic nephroma occurring after transfer of a cryopreserved embryo. Hum Reprod. 1999; 14:1324–1327.
12. Kim CH, Kim YH, Cho MK, Kim KM, Ha JA, Joo EH, et al. A case of fetal congenital mesoblastic nephroma with oligohydramnios. J Korean Med Sci. 2007; 22:357–361.
13. Vujanic GM, Delemarre JF, Moeslichan S, Lam J, Harms D, Sandstedt B, et al. Mesoblastic nephroma metastatic to the lungs and heart: another face of this peculiar lesion: case report and review of the literature. Pediatr Pathol. 1993; 13:143–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download