Abstract
Endometrial cancer is the most common malignancy of the female genital tract. The cancer spreads by direct extension, transtubal dissemination, lymphatic dissemination, and/or by hematogenous spread, usually results in lung metastasis, but may less commonly involve liver, brain, and bone. Here, we describe a patient with stage IA endometrial cancer who developed liver recurrence 17 months after surgery.
Endometrial cancer is the most common gynecologic malignancy in the United States [1], and the incidence of the disease is increasing rapidly in Korea, where it is the third most common malignancy in women, affecting approximately 862 patients per year [2]. The number of patients with recurrent endometrial cancer is also increasing, and currently 10% to 15% of patients with early stage endometrial cancer experience recurrence [3]. Endometrial cancer spreads by direct extension, transtubal dissemination, lymphatic dissemination, and hematogenous spread, the latter of which most commonly results in lung metastasis, but liver, brain, and bone are also less commonly involved.
Here, we report a case of early endometrial cancer with focal myometrial invasion that recurred in liver.
In May 2007, a 69-year-old Korean woman presented at our emergency room with a chief complaint of vaginal bleeding, which had started a few days earlier. She was healthy at birth and had no history of underlying disease or a clotting abnormality. In addition, there was no history of oral medication, pregnancy, or any other gynecological/surgical problem. Transvaginal ultrasound revealed hematometra and necrotic materials in the uterine cavity. Abdomino-pelvic computed tomography (CT) revealed hematometra occupying the whole distended uterine cavity and ascites (Fig. 1). An endometrial biopsy obtained via dilation and curettage showed endometrioid adenocarcinoma of the uterus, grade 2 (Fig. 2). Her serum CA-125 level was 514.0 U/mL (normal range, <35.0 U/mL). The patient subsequently underwent surgical staging, including total abdominal hysterectomy, bilateral salpingooophorectomy, and pelvic and para-aortic lymphadenectomy. Final pathological findings revealed 2009 International Federation of Obstetrics and Gynecology stage IA endometrioid adenocarcinoma, grade 2, with focal myometrial invasion. Peritoneal washings were negative for malignant cells. The patient was then treated with adjuvant brachytherapy to a total dose of 3,000 cGy in 6 fractions of 500 cGy each. After treatment, serum CA-125 had returned to the normal range.
The patient remained under close follow-up every three month (CA-125 and pelvic exam) and was without any evidence of recurrent disease until November 2008. At that time, her serum CA-125 level was found to have increased to 93.03 U/mL, and a subsequent F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) scan revealed recurrent mass with abnormal FDG uptake in the liver. A liver biopsy revealed recurrent adenocarcinoma. The patient then underwent six cycles of a 3 weekly paclitaxel and carboplatin chemotherapy regimen. However, 8 months after completing chemotherapy, serum CA-125 was elevated and abdomino-pelvic CT revealed multiple recurrences. Second-line chemotherapy with 5-FU and cisplatin was attempted, but her response was poor, and her general condition became aggravated. The patient is currently under hospice care, and is being treated as a terminal cancer patient.
The presented case represents the unusual recurrence of endometrioid carcinoma in the liver. The most common site of endometrioid carcinoma recurrence is the vaginal cuff, and approximately 75% of recurrences occur at this site without adjuvant therapy. However, although adjuvant radiotherapy reduces locoregional recurrence, the vaginal cuff still remains the most common site of relapse within the pelvis [3,4]. Sohaib et al. [5] reported that the most frequent sites of relapse by cross-sectional imaging are in lymph nodes, the vaginal vault, peritoneum, and lung. Hydronephrosis secondary to disease within the pelvis is also a frequent finding. Less frequent sites of distant hematogenous spread include liver, spleen, pancreas, bone, brain, abdominal wall, and skeletal muscle [5].
The etiology of recurrence in our case may be posited to have been due to tumor cells left behind after initial surgery that survived adjuvant radiation therapy. Another possibility to consider is the malignant transformation of endometriosis [6]. The de novo development of endometrioid carcinoma from ectopic endometriosis is also a possibility [7], although carcinogenesis from endometriosis is rare [8]. Our patient had no known history of endometriosis and no evidence of ectopic uterine tissue by original pathology following hysterectomy, and thus, this scenario seems less than likely.
Patients with stage 1A and grade 1 to 2 tumors are considered low risk, with at most a 5% chance of locoregional recurrence [9]. Isolated vaginal recurrence is the most common type of treatment failure after surgery in patients with early stage endometrial cancer, but our patient unusually experienced liver recurrence. Unfortunately, no consensus exists on the management of this patient population. Current guidelines are broad and include tumor-directed radiotherapy, and/or chemotherapy, and/or surgical resection. Risk factors regarding the original diagnosis and disease recurrence (e.g., location of recurrence, prior therapy) are evaluated on an individual basis to tailor treatment plans. For example, patients treated with radiation initially would routinely be offered chemotherapy as a component of the treatment for recurrent disease.
The likelihood of liver recurrence in low risk patients with stage 1A endometrial cancer is quite low. Further stratification of patients that experience recurrence according to site of recurrence, prior radiation therapy, and initial disease characteristics may help focus treatment guidelines for these patients.
Figures and Tables
Fig. 1
(A) Abdomino-pelvic computed tomography showed fluid filled distended uterus within enhancing nodular masses. (B) The tumor via endometrium consist of moderate differentiated endometrioid adenocarcinoma, grade 2 (H&E, ×100).
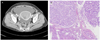
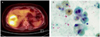
Fig. 2
(A) Positron emission tomography-computed tomography showed recurrent mass with abnormal fluorodeoxyglucose uptake in the liver. (B) The smear of the aspirate from the hepatic lesion is markedly hypocellularity, disclosing a few loose clusters and cytological features show possibility of adenocarcinoma (PAP stain, ×200).
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
2. SOG Gynecologic Oncology Committee. Annual report of gynecologic cancer registry program in Korea for 2004 (Jan. 1st, 2004-Dec. 31st, 2004). Korean J Obstet Gynecol. 2007; 50:28–78.
3. Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev. 2012; 4:CD003916.
4. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000; 355:1404–1411.
5. Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007; 62:28–34.
6. Kobayashi S, Sasaki M, Goto T, Asakage N, Sekine M, Suzuki T, et al. Endometrioid adenocarcinoma arising from endometriosis of the rectosigmoid. Dig Endosc. 2010; 22:59–63.
7. Hoang CD, Boettcher AK, Jessurun J, Pambuccian SE, Bullard KM. An unusual rectosigmoid mass: endometrioid adenocarcinoma arising in colonic endometriosis: case report and literature review. Am Surg. 2005; 71:694–697.
8. Schneider A, Touloupidis S, Papatsoris AG, Triantafyllidis A, Kollias A, Schweppe KW. Endometriosis of the urinary tract in women of reproductive age. Int J Urol. 2006; 13:902–904.
9. Straughn JM Jr, Huh WK, Kelly FJ, Leath CA 3rd, Kleinberg MJ, Hyde J Jr, et al. Conservative management of stage I endometrial carcinoma after surgical staging. Gynecol Oncol. 2002; 84:194–200.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download