Abstract
Objective
The objectives of this study were to determine the efficacy of NV-196, a synthetic isoflavone derivative, as a chemosensitizer in chemoresistant CP70 and R182 epithelial ovarian cancer (EOC) cells and to characterize the mechanism behind its sensitizing effect.
Methods
EOC cells were treated with tenfold dilutions of NV-196 (0.1 to 10 μg/mL) for 24 and 48 hours. Cell viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay. Apoptosis was assessed by Caspase-Glo assays and apoptotic cascade X-linked inhibitor of apoptosis protein (XIAP), caspase-2 and Bid were characterized by Western blot analyses.
Results
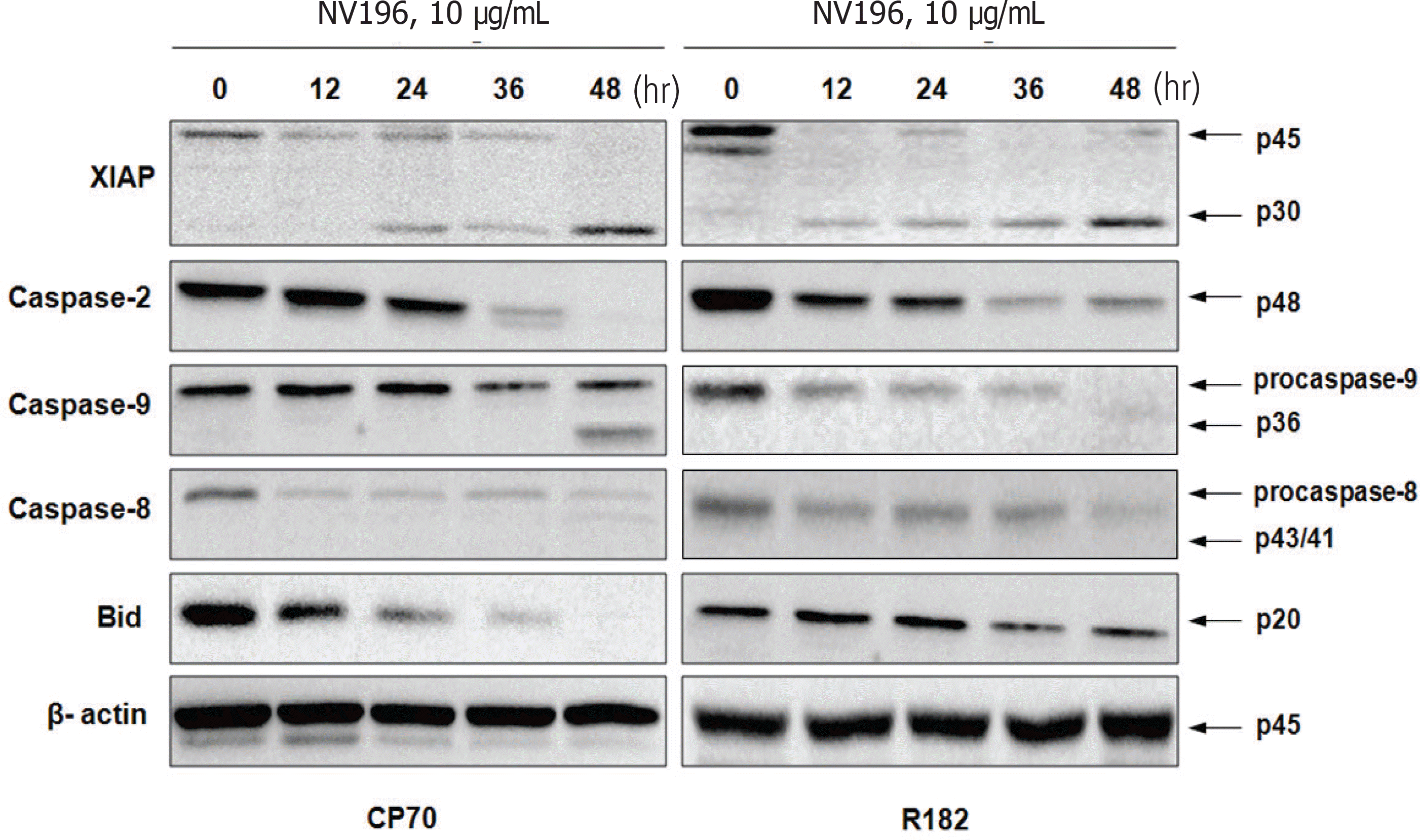
As a monotherapy, NV-196 showed decreased cell viability in a time- and dose-dependent manner in both CP70 and R182 cells. A significant increase in caspase-3 activity was observed in both cells. Caspase-8 and -9 activation were also observed. Western blots demonstrated Bid and caspase-2 activation and cleavage of XIAP. NV-196 enhances the cytotoxic effects of carboplatin and paclitaxel.
REFERENCES
1. National Cancer Control Institute (KR). 2008 national cancer statistics. Goyang: National Cancer Center;2010.
2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.

3. Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007; 82:751–70.

4. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000; 256:42–9.

5. Gross SA, Newton JM, Hughes FM Jr. Decreased intracellular potassium levels underlie increased progesterone synthesis during ovarian follicular atresia. Biol Reprod. 2001; 64:1755–60.
6. Saif MW, Tytler E, Lansigan F, Brown DM, Husband AJ. Flavonoids, phenoxodiol, and a novel agent, triphendiol, for the treatment of pancreaticobiliary cancers. Expert Opin Investig Drugs. 2009; 18:469–79.

7. Alvero AB, Brown D, Montagna M, Matthews M, Mor G. Phenoxodiol-Topotecan co-administration exhibit significant anti-tumor activity without major adverse side effects. Cancer Biol Ther. 2007; 6:612–7.

8. Alvero AB, O'Malley D, Brown D, Kelly G, Garg M, Chen W, et al. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006; 106:599–608.

9. Sapi E, Alvero AB, Chen W, O'Malley D, Hao XY, Dwipoyono B, et al. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004; 14:567–78.

10. Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, et al. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987; 47:414–8.
11. Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, et al. Phenoxodiol–an isoflavone analog–induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003; 22:2611–20.
12. Peter ME, Scaffidi C, Medema JP, Kischkel F, Krammer PH. The death receptors. Results Probl Cell Differ. 1999; 23:25–63.

13. Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998; 94:481–90.

14. Alvero AB, Chen W, Sartorelli AC, Schwartz P, Rutherford T, Mor G. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) induces apoptosis in ovarian cancer cells. J Soc Gynecol Investig. 2006; 13:145–52.
15. Fraser M, Leung BM, Yan X, Dan HC, Cheng JQ, Tsang BK. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003; 63:7081–8.
16. Li J, Sasaki H, Sheng YL, Schneiderman D, Xiao CW, Kotsuji F, et al. Apoptosis and chemoresistance in human ovarian cancer: is Xiap a determinant? Biol Signals Recept. 2000; 9:122–30.

17. Tong QS, Zheng LD, Wang L, Zeng FQ, Chen FM, Dong JH, et al. Downregulation of XIAP expression induces apoptosis and enhances chemotherapeutic sensitivity in human gastric cancer cells. Cancer Gene Ther. 2005; 12:509–14.

18. McManus DC, Lefebvre CA, Cherton-Horvat G, St-Jean M, Kandimalla ER, Agrawal S, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004; 23:8105–17.

19. Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001; 21:3604–8.

20. Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008; 7:1036–46.
21. Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006; 13:179–88.

22. Johnson DE, Gastman BR, Wieckowski E, Wang GQ, Amoscato A, Delach SM, et al. Inhibitor of apoptosis protein hILP undergoes caspase-mediated cleavage during T lymphocyte apoptosis. Cancer Res. 2000; 60:1818–23.
23. Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for cas- pase-2 in stress-induced apoptosis before mitochondrial per-meabilization. Science. 2002; 297:1352–4.
Fig. 1.
(A, B) NV-196 decreases the viability of epithelial ovarian cancer (EOC) cells (CP70 and R182). The viability (in percentage, normalized to untreated cells) of EOC cells after treatment with increasing concentration of NV-196 for 24 and 48 hours. Data were compiled from at least three independent experiments, each done in triplicate (∗p<0.05). (C, D) CP70 and R182 cells were pretreated with 10 μg/mL NV-196 for 8 hours and then treated with carboplatin or paclitaxel for 24 hours. Cell viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (∗p<0.05).
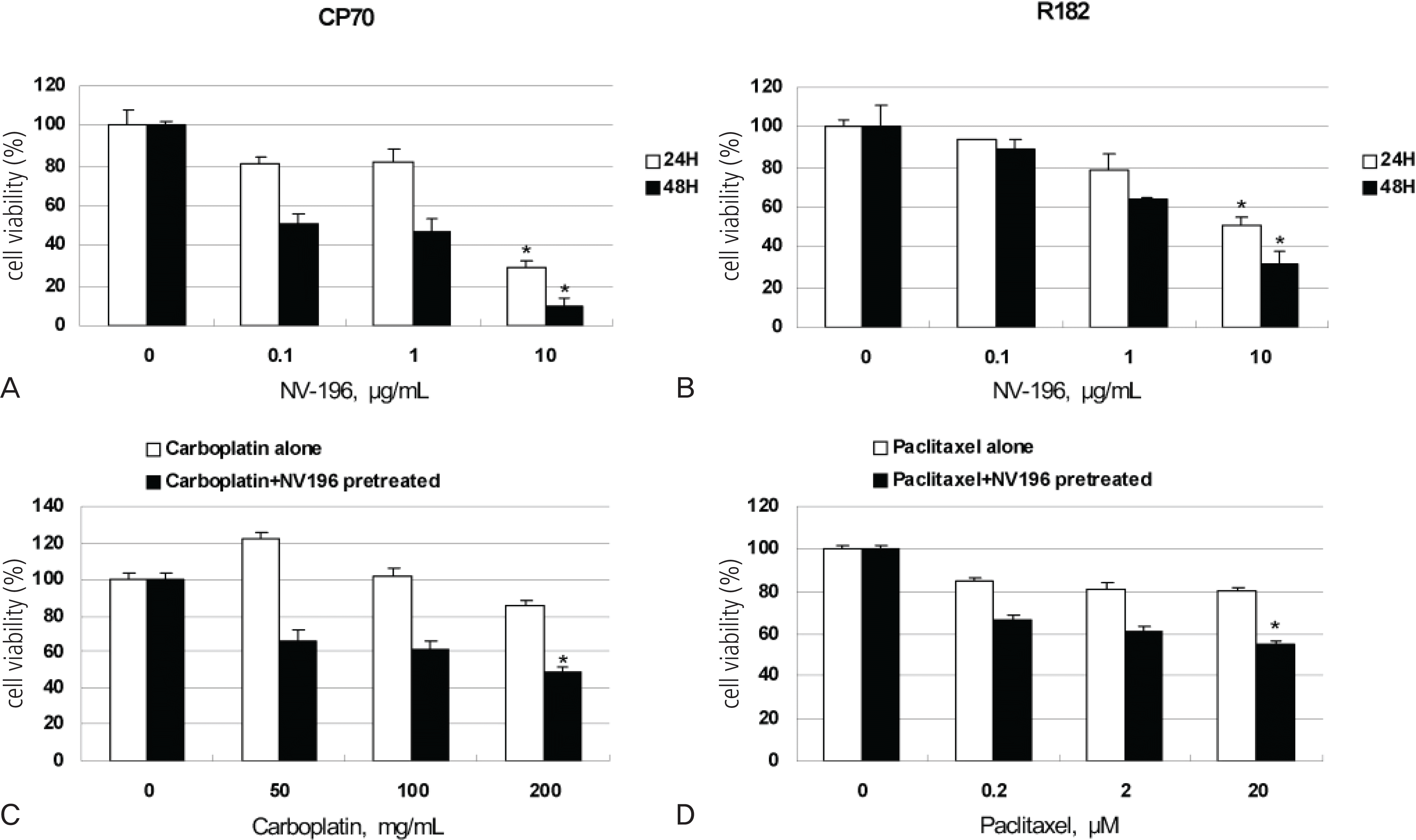
Fig. 2.
(A) Phase-contrast images of NV-196 induced apoptosis in CP70 and R182 cells (×200). Both cells were treated with 10 μg/mL NV-196 for 24 and 48 hours. (B) Hoechst dye staining of apoptotic nuclei (×400), (a) non-treated control, (b) NV-196 treated CP70 cells, 10 μg/mL, 24 hours. NT: non-treated control.
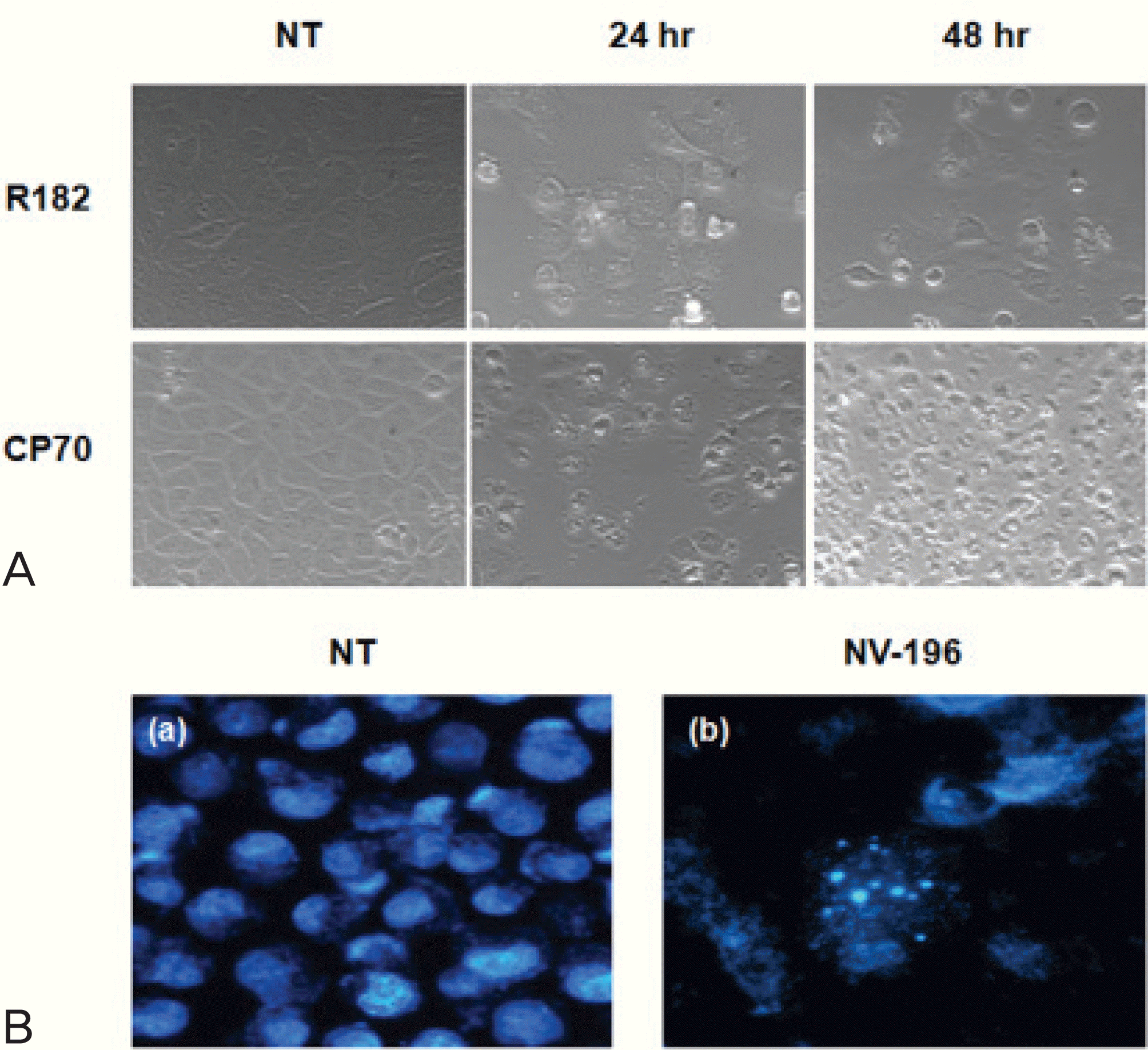
Fig. 3.
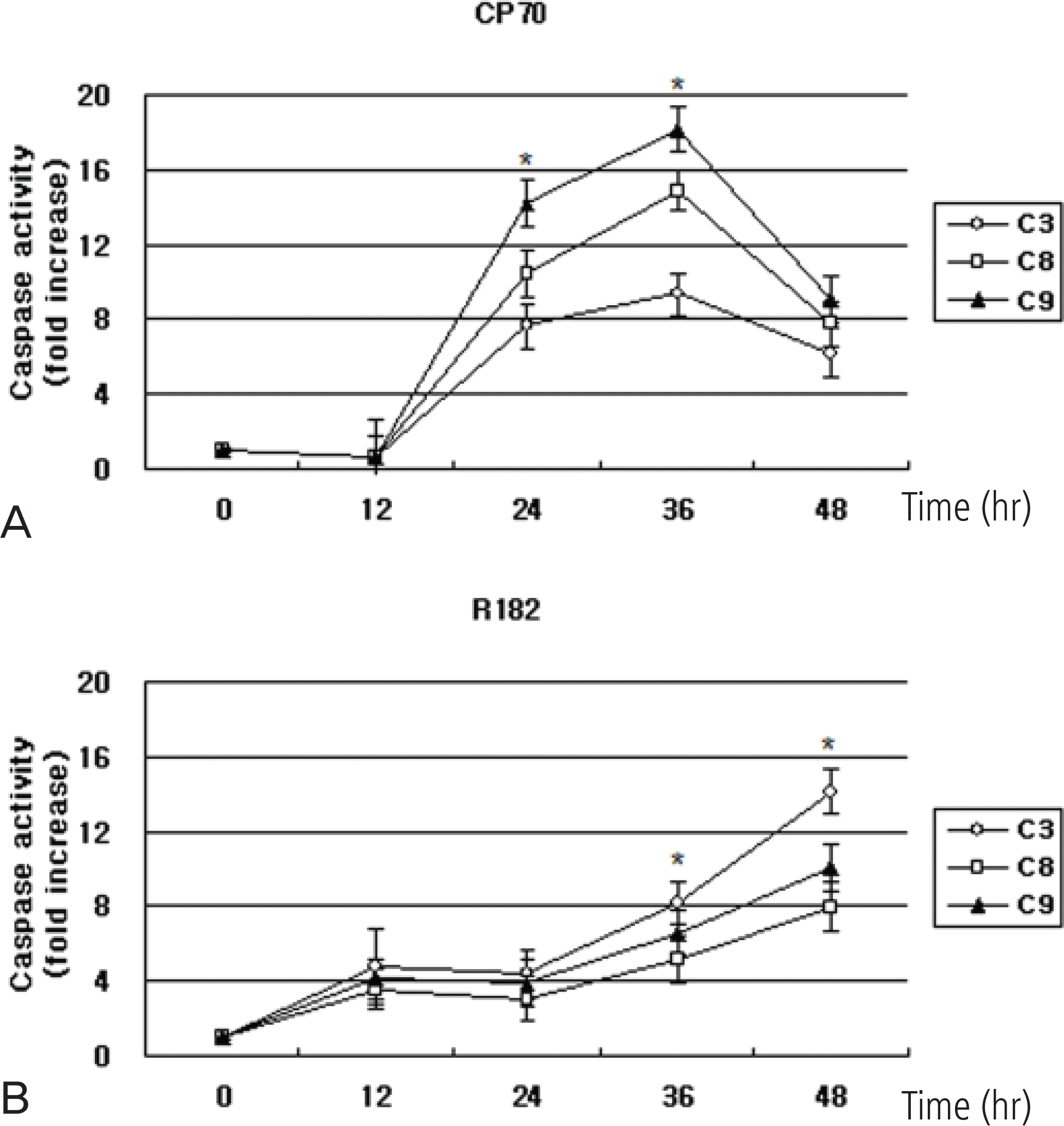
Relative caspase-3, -8, -9 activity in CP70 and R182 cells. Cells were exposed to 10 μg/mL NV-196 for 12, 24, 36 and 48 hours. Caspase activity was measured by Caspase-Glo assays as described in Materials and Methods. Y axis: caspase activity fold increase from control. The data were compiled from at least three independent experiments (∗p<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download