Abstract
Objective
We designed this study to explore the pregnancy outcomes in women with hyperglycemia according to their prepregnancy body mass index (BMI) and to identify risk factors of poor pregnancy outcomes.
Methods
A total of 1,056 pregnant women, who took a standard 100 g oral glucose tolerance test, were recruited between July 1, 2007 and December 31, 2009. The participants were stratified into 3 groups (group 1 [BMI < 18.5 kg/m2], group 2 [BMI 18.5-24.9 kg/m2], and group 3 [obese] [BMI ≥ 25 kg/m2]) based on their prepregnancy BMI following the World Health Organization Asia-Pacific guidelines.
Results
Older age and multi-parity, and family history of diabetes were significantly higher in the obese group. Development of hypertension and gestational diabetes mellitus were also significantly increased with obesity. Maternal weight gain, however, was inversely correlated pattern with prepregnancy BMI. Poor pregnancy outcomes are increased with older age, multi parity, gestational ages at delivery, increased prepregnancy BMI, maternal high glucose status and weight gain rate. Particularly, prepregnancy BMI had higher risk than maternal hyperglycemia on macrosomia (odd ratio [OR] 5.0, 95% confidence intervals [CI] 2.28-11.02 vs. OR 3.0, 95% CI 1.63-5.85), and on primary cesarean section rate (OR 2.5, 95% CI 1.46-4.46 vs. OR 1.6, 95% CI 1.14-2.43).
Conclusion
Pregnant women with obesity are more likely to have poor pregnancy outcomes than pregnant women without obesity. Therefore, with prepregnancy BMI considered, effective management during pregnancy should be designed and intervention trials are needed to identify individuals at risk before developing hyperglycemia.
Although the gestational period is considerably short in women's lifespan, pregnancy has substantial influences to both mother and her offspring. Studying weight gain during pregnancy has been one of the hot topics in obstetrics for the last decades [1-5]. In these days overweight or obesity in women of reproductive age is rapidly increasing worldwide and they are more likely to gain excess weight during pregnancy [1-3,6]. Overweight or obesity is considered as a common high-risk obstetric condition along with gestational diabetes, hypertensive disorder during pregnancy, operative delivery and poor fetal outcomes [7-10].
In 2009, the American Institute of Medicine (IOM) issued guidelines for appropriate weight gain, assigning target ranges based on maternal prepregnancy body mass index (BMI). The recommended ranges of gestational weight gain are 12.5-18 kg for underweight women (BMI < 18.5 kg/m2), 11.5-16.0 kg for normal weight women (BMI 18.5-24.9 kg/m2), 7.0-11.5 kg for overweight women (BMI 25-29.9 kg/m2), and 5.0-9.0 kg for obese women (BMI ≥ 30 kg/m2) [11]. The recommended rates of weight gain during 2nd and 3rd trimester are 1 lb/week for underweight women, 1 lb/week for normal weight women, 0.6 lb/week for overweight women and 0.5 lb/week for obese women.
Normally pregnant women are in diabetic condition during pregnancy because of their babies. The elevation of maternal glycemia is a consequence to a rise in maternal insulinemia, which facilitate fetal growth [12]. Increased maternal BMI during pregnancy and glucose status contribute to offspring birth size and birth outcomes. Although a positive association between prepregnancy BMI and disturbance in glucose metabolism has been established [13-15], it is not clearly known if prepregnancy BMI can affect the pregnancy outcomes of women with at risk of gestational diabetic condition.
In this context, we designed this study to explore the pregnancy outcomes in women at risk of hyperglycemic state with respect to prepregnancy BMI and to identify the risk factors of poor pregnancy outcome.
This study used data from pregnant women whose 100 g oral glucose tolerance (OGTT) test was preformed between July 1, 2007 and December 31, 2009 at CHA Gangnam Medical Center (Seoul, Korea). Pregnant women at risk of hyperglycemia were suggested by a positive result of 50 g glucose challenge test followed by a confirmatory 100 g fasting glucose 3-hour tolerance test (OGTT). This study was approved by the Institutional review board of CHA Medical Center, CHA University. Data of 1,215 pregnant women were collected, among which 159 participants with twin pregnancies, fetal anomalies, hypertensive disorders before pregnancy, preexisting diabetes and other diseases, and missing medical records were excluded.
Prepregnancy BMI (kg/m2) were calculated using self-reported prepregnancy weight and height. The participants (n = 1,056) were divided into three groups: group 1 (BMI < 18.5 kg/m2, n = 178); group 2 (BMI 18.5-24.9 kg/m2, n = 769); and group 3 (BMI ≥ 25 kg/m2, n = 109) according to the guidelines of World Health Organization (WHO) Asia-Pacific [16,17] and considering IOM [11]. In the current study, we designated the group 3 as obese group, and other two groups as non-obese group. The overweight prepregnancy BMI in the WHO Asia-Pacific guidelines corresponds to normal prepregnancy BMI in the IOM guidelines. Therefore, so we grouped participants with BMI between 18.5 kg/m2 and 25 kg/m2 into a single group (group 2).
At the first clinic visit before gestational 10 weeks, participants reported pre-pregnant weight, height and their medical history, which were double checked by their obstetricians. Gestational age was calculated based on her last menstrual period and adjusted by sonographic findings at the first clinic visit. Gestational weights were recorded at second trimester glycemic screening test between 24 and 28 weeks and at delivery. We examined blood pressure and urine analysis due to evaluation of pregnancy complications such as hypertension and proteinuria. Additional data were also collected on maternal age, parity, family history of diabetes or hypertension.
At 24-28 weeks, all participants who had more than 140 mg/dL of 1-hour glucose level in the venous blood sample on a routine glycemic screening as a non-fasting oral glucose challenge test, underwent additional 100 g fasting glucose 3-hour tolerance test. Normal OGTT results were based on Carpenter and Coustan criteria (<95 mg/dL) at fasting state, <180 mg/dL at 1 hour, <155 mg/dL at 2 hours, <140 mg/dL at 3 hours [18]. Gestational diabetes mellitus (GDM) was defined by the presence of at least two abnormal OGTT glucose values. If participating women were diagnosed with GDM, they received diet and exercise instructions, and insulin therapy, if needed.
In terms of pregnancy outcomes, preterm delivery was defined as delivery at less than 37 weeks of gestation; macrosomia was defined as birth weight of 4,000 g or greater; Large for gestational age (LGA) was defined as birth weight more than 90 percentile; small for gestational age (SGA) was defined as birth weight less than 10 percentile; the conditions of primary cesarean section included from failure to progress, fetal malpresentation, and past history of uterus operation.
Data were demonstrated using descriptive statistics as mean ± standard deviation and percentage in the order of group 1, group 2 and group 3 followed by P-values from statistical tests, unless otherwise stated. The differences of variables between and among groups were analyzed with Fisher's exact test and one way analysis of variance (ANOVA) test with Bonferroni post-Hoc test Odd ratio (OR) with 95% confidence intervals (CI) was calculated by multiple logistic regression analysis. The level of statistical significance was considered a P-value less than 0.05. For the statistical analysis, SPSS ver. 17 (SPSS Inc., Chicago, IL, USA) was used..
Total 1,056 pregnant women met our inclusion criteria. Their characteristics in the different prepregnancy BMI groups are listed in Table 1. Age was significantly different among the three groups (31.8 ± 3.7 [group 1], 32.9 ± 3.8 [group 2], 34.0 ± 3.7 [group 3] years, ANOVA P < 0.001); prepregnancy obese women were older than other groups with statistical significance (P < 0.001 and P = 0.02 compared to groups 1 and 2, respectively). Multiparity was also different among the groups (1.2 ± 0.5, 1.4 ± 0.6, 1.7 ± 0.9, ANOVA P < 0.001) with obese women having the higher multiparity than other groups (all post-hoc P < 0.001). Family history of diabetes was not significantly different (19%, 19%, 28%, Fisher's test P = 0.068), but the obese group showed the highest rate. Although the three groups had mean blood pressures in normal ranges, the obese group showed significantly higher blood pressures (systolic pressure, 108.5 ± 10.9 mm Hg, 114.0 ± 11.4 mm Hg, 120.8 ± 12.7 mm Hg; ANOVA P < 0.001; diastolic pressure, 64.7 ± 7.8 mm Hg, 67.3 ± 7.8 mm Hg, 72.8 ± 9.0 mm Hg; ANOVA P < 0.001) (all post-hoc P < 0.001) and higher incidence of hypertension during pregnancy (1%, 2%, 13%; Fisher's test P < 0.001, all pair-wise P < 0.01). Development of GDM was also significantly more frequent in obese women (15%, 24%, 43%; Fisher's test P < 0.001 and all pair-wise P < 0.001).
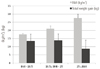
At the time of 100 g glucose tolerance test, hemoglobin A1c. (5.1 ± 0.4%, 5.4 ± 0.5%, 5.5 ± 0.6%; ANOVA P < 0.001 and all post-hoc P < 0.01) and fasting glucose levels (77.3 ± 6.9 mg/dL, 80.7 ± 9.6 mg/dL, 85.7 ± 12.2 mg/dL; ANOVA P < 0.001 and all post-hoc P < 0.001) were significantly increased in the obese group. Total weight gains during pregnancy was in the IOM-recommended ranges (13.50 ± 4.29 kg, 12.15 ± 4.02 kg, 8.30 ± 5.35 kg) but showed a decreasing pattern with increasing maternal obesity (Fig. 1). As illustrated in Fig. 2, the non-obese groups (group 1 and group 2) had higher weight gain rates after 50 g OGCT (group 1: from 3.2 ± 1.3 to 4.0 ± 1.9 100 g/week and group 2: from 3.1 ± 1.3 to 3.9 ± 2.0 100 g/week), while the obese group had similar weight gain rates before and after 50 g OGCT (3.0 ± 1.3 and 3.0 ± 2.5 100 g/week).
Pregnancy outcomes of these groups are presented in Table 2. Gestational ages at delivery were not different among the groups, but fetal birth weight increased significantly across the groups (3,214.2 ± 383.1 g, 3,325.7 ± 410.0 g, 3,445.6 ± 501.6 g; ANOVA P < 0.001 and all post-hoc P < 0.01). The percentages of macrosomia (1%, 5%, 14%; Fisher's test P < 0.001, all pair-wise P < 0.001) and LGA babies (5%, 9%, 25%; Fisher's test P < 0.001, all pairwise P < 0.001) were significantly higher in the obese group. On the other hands, the incidences of SGA babies decreased as prepregnancy BMI increased (17%, 9%, 7%; Fisher's test P = 0.008), but group 3 (obese group) was significantly different only from group 1 (P = 0.009), not group 2 (P = 0.380). Although no case of preterm delivery was found in the obese group unlike other groups, the difference was not statistically significant (P = 0.293). Frequency of low apgar score (< 7) was also not different among the groups (P = 0.765). Primary cesarean section rate was higher in the obese group than the other two groups (27%, 32%, 49%; Fisher's test P = 0.002, all pair-wise P < 0.001).
The results of risk factor analyses on the pregnancy outcomes are summarized in Table 3. The risk of macrosomia, LGA, primary cesarean section increased with older age, higher prepregnancy BMI, multiparity, higher frequency of GDM development, higher total weight gain and later gestational age at delivery. Particularly for macrosomia, prepregnancy BMI was a higher risk factor than maternal hyperglycemia (OR 5.0, 95% CI 2.28-11.02 vs. OR 3.0, 95% CI 1.63-5.85). Other factors including LBW, and low APGAR score were not affected by total weight gain and prepregnancy BMI, as diabetic risk (GDM) did not affect SGA and LBW and low apgar score. Primary cesarean section was affected by both prepregnancy BMI and maternal diabetic condition (OR 2.5, 95% CI 1.46-4.46 vs. OR 1.6, 95% CI 1.14-2.43).
Over the last decades, the population of childbearing women has experienced dramatic changes such as multiple pregnancies, older age, heavier body weights. These changes have imposed higher health risks in both mothers and their offspring. Therefore, the Institute of Medicine has proposed the guidelines of pregnancy weight from the beginning of conception through the first year after delivery to ensure welfare of infant and the health of mother [11].
To address whether IOM recommendations are advisable and safe for pregnant women, many observational studies have compared the risk for pregnancy outcomes with respect to gestational weight gain with IOM criteria [19-21]. Although this study was retrospectively performed, our study confirmed that high prepregnancy BMI is highly associated with adverse pregnancy outcomes, more than the diabetic condition and any other important risk factors known to affect poor pregnancy outcomes in Korean women.
Throughout gestation pregnant women physiologically go through both weight gain and diabetic condition. In multiple studies, maternal obesity has been identified as one of the most common factors for high risk obstetric conditions such as gestational diabetes, hypertensive disorder in pregnancy, fetal death, and macrosomia and cesarean delivery in various ethnic groups [8,10,22]. The Asian population, as in the current study, has different characteristics of diabetes and obesity from other ethnic groups. Asians tend to have a higher percentage of body fat than Caucasians at the same BMI and have a greater tendency to accumulate adipose tissue in visceral rather than subcutaneous compartments compare to most other ethnic groups. Asians also tend to have a lower capacity to secrete insulin and are less able to compensate for insulin resistance than other ethnic groups [23,24].
The importance of prepregnancy BMI affecting pregnancy outcomes has been widely studies, particularly with respect to the offspring adiposity, insulin resistance and maternal diabetes incidence [4,5,13,25-28]. GDM complicated 1-14% of pregnancy in United States [29] and 2-5% of all pregnant women reportedly developed GDM in Korea [30]. In the current study, Hba1c was significantly higher and fasting glucose level was also increased in the obese women. A significantly higher percentage of the obese women (approximately 43%) developed GDM in our study, while the other two groups developed GDM in 15% and 24% of women.
Glucose travels freely from mother to fetus, but maternal insulin does not. In general, increased glucose induces maternal insulin resistance and elevated maternal amino acid and fatty acids, which are the major stimulators of fetal adiposity and fetal growth [12,25,31]. In 1950s, Pedersen et al.[32] suggested that in the offspring of mothers with diabetes, excess fetal insulin production was the primary factor in promoting fetal overgrowth. In our study, maternal diabetic state had three fold risks on macrosomia and high prepregnancy BMI had five times higher influence on macrosomia. These results parallel others' finding that excessive fetal growth, one of the well-characterized adverse outcomes, is increased about three fold in diabetic pregnancy [33-35].
Fetal birth weight was significantly heavier in the obese group, which has similar maternal weight gain as other groups before the 50 g OGCT test; however, the gain after the 50 g OGCT test was much smaller than the other groups. This resulted in significantly lower weight gain in the obese group, which is consistent with others' finding that gestation weight is inversely correlated with prepregnancy BMI [36,37]. Although the maternal weight gain was within the IOM's recommendation for each group, fetal birth weight was still highly associated with prepregnancy BMI In the present study, maternal prepregnancy BMI affected macrosomia more substantially than maternal diabetic condition, which is also consistent with other studies of obesity in pregnant women with GDM [38,39]. Although obesity is a high risk factor for maternal and neonatal morbidity and mortality, it is a preventable risk factor by proper management [7].
Taken together, our study suggests prepregnancy BMI state may affect maternal insulin resistance and maternal glycemic status, eventually have a substantial impact on pregnancy outcomes. In order to reduce poor perinatal outcomes and state effective pregnancy managing, proper weight management ensuring appropriate weight gain according to their prepregnancy BMI during pregnancy is needed. Maternal prepregnancy condition could have a substantial impact on public health, especially maternal prepregnancy BMI and maternal weight gain might be a pointer for differences in maternal metabolism that are independently associated with adverse pregnancy outcomes. Therefore, lifestyle interventions should reduce perinatal morbidity and mortality. Further studies on the maternal weight before and during pregnancy are needed to better understand this phenomenon.
Figures and Tables
Fig. 2
Maternal weight gain rate before and after oral glucose challenge test. Among these groups there were significant different weight gain (P <0.001). OGCT, oral glucose challenge test; BMI, body mass index.
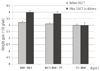
Table 3
Risk factor analysis on pregnancy outcomes

LGA, large for gestational age; CS, cesarean section; RCS, repeat cesarean section; LBW, low borth weight; SGA, small for gestational age; GA, gestational age; BMI: body mass index; GDM, gestational diabetes mellitus; OGCT, oral glucose challenge test.
a is P value < 0.05, with statistical significance; bindicates primi-parity, while others are multiparity.
References
1. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004. 291:2847–2850.
2. Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. Am J Obstet Gynecol. 2006. 194:e32–e34.
3. Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obesity (Silver Spring). 2007. 15:986–993.
4. Jensen DM, Damm P, Sørensen B, Mølsted-Pedersen L, Westergaard JG, Ovesen P, et al. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstet Gynecol. 2003. 189:239–244.
5. Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J Matern Fetal Med. 1998. 7:190–193.
6. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002. 288:1723–1727.
7. Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normalweight women: effects of gestational weight change. Obstet Gynecol. 1996. 87:389–394.
8. Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstet Gynecol. 1998. 91:97–102.
9. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001. 91:436–440.
10. Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001. 25:1175–1182.
11. Institute of Medicine and National Research Council. Weight gain during pregnancy:reexamining the guidelines. 2009. Washington (DC): Natinal Academy Press.
12. Sosenko IR, Kitzmiller JL, Loo SW, Blix P, Rubenstein AH, Gabbay KH. The infant of the diabetic mother: correlation of increased cord C-peptide levels with macrosomia and hypoglycemia. N Engl J Med. 1979. 301:859–862.
13. Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with metaanalysis. Obes Rev. 2009. 10:194–203.
14. Jensen DM, Sorensen B, Feilberg-Jørgensen , Westergaard JG, Beck-Nielsen H. Maternal and perinatal outcomes in 143 Danish women with gestational diabetes mellitus and 143 controls with a similar risk profile. Diabet Med. 2000. 17:281–286.
15. Virally M, Laloi-Michelin M, Meas T, Ciraru N, Ouled N, Medeau V, et al. Occurrence of gestational diabetes mellitus, maternal and fetal outcomes beyond the 28th week of gestation in women at high risk of gestational diabetes. A prospective study. Diabetes Metab. 2007. 33:290–295.
16. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000. 894:i–xii. 1–253.
17. Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005. 94:1–12.
18. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007. 30:Suppl 2. S251–S260.
19. Beyerlein A, Lack N, von Kries R. Within-population average ranges compared with Institute of Medicine recommendations for gestational weight gain. Obstet Gynecol. 2010. 116:1111–1118.
20. DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007. 110:745–751.
21. Lederman SA. Optimal gestational weight gain must not be determined from adverse birth weight outcomes defined only as the total percentage of infants born small- or large-forgestational-age. Am J Clin Nutr. 2010. 91:819–821.
22. Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond). 2008. 32:495–501.
23. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009. 301:2129–2140.
24. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010. 375:408–418.
25. Ong KK, Diderholm B, Salzano G, Wingate D, Hughes IA, Mac-Dougall J, et al. Pregnancy insulin, glucose, and BMI contribute to birth outcomes in nondiabetic mothers. Diabetes Care. 2008. 31:2193–2197.
26. Driul L, Cacciaguerra G, Citossi A, Martina MD, Peressini L, Marchesoni D. Prepregnancy body mass index and adverse pregnancy outcomes. Arch Gynecol Obstet. 2008. 278:23–26.
27. Murakami M, Ohmichi M, Takahashi T, Shibata A, Fukao A, Morisaki N, et al. Prepregnancy body mass index as an important predictor of perinatal outcomes in Japanese. Arch Gynecol Obstet. 2005. 271:311–315.
28. López-Bermejo A, Petry CJ, Díaz M, Sebastiani G, de Zegher F, Dunger DB, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab. 2008. 93:1501–1505.
29. American Diabetes Association. Standards of medical care in diabetes: 2008. Diabetes Care. 2008. 31:Suppl 1. S12–S54.
30. Jang HC, Cho YM, Park KS, Kim SY, Lee HK, Kim MY, et al. Pregnancy outcome in Korean women with gestational diabetes mellitus diagnosed by the Carpenter-Coustan criteria. J Korean Diabetes Assoc. 2004. 28:122–130.
31. Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia. 2002. 45:991–996.
32. Pedersen J, Bojsen-Møller B, Poulsen H. Blood sugar in newborn infants of diabetic mothers. Acta Endocrinol (Copenh). 1954. 15:33–52.
33. Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992. 15:1251–1257.
34. Geifman-Holtzman O, Machtinger R, Spiliopoulos M, Schiff E, Koren-Morag N, Dulitzki M. The clinical utility of oral glucose tolerance test at term: can it predict fetal macrosomia? Arch Gynecol Obstet. 2010. 281:817–821.
35. Conway DL, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998. 178:922–925.
36. Jensen DM, Ovesen P, Beck-Nielsen H, Mølsted-Pedersen L, Sørensen B, Vinter C, et al. Gestational weight gain and pregnancy outcomes in 481 obese glucose-tolerant women. Diabetes Care. 2005. 28:2118–2122.
37. Tovar A, Must A, Bermudez OI, Hyatt RR, Chasan-Taber L. The impact of gestational weight gain and diet on abnormal glucose tolerance during pregnancy in Hispanic women. Matern Child Health J. 2009. 13:520–530.
38. Kang CH, Kim MR, Choi MY, Kang EJ, Kim HJ, Seo SS. Clinical comparison of maternal characteristics and pregnancy outcomes between gestational diabetes and general obstetric population. Korean J Obstet Gynecol. 2001. 44:478–485.
39. Park JE, Park S, Daily JW, Kim SH. Low gestational weight gain improves infant and maternal pregnancy outcomes in overweight and obese Korean women with gestational diabetes mellitus. Gynecol Endocrinol. 2011. 27:775–781. DOI: 10.3109/09513590.2010.540597.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download