Abstract
Allergic bronchopulmonary mycosis (ABPM) develops mainly in patients with asthma or cystic fibrosis via types I and III hypersensitivity reactions to filamentous fungi. Aspergillus spp., especially Aspergillus fumigatus, is the major causative fungus because of its small conidia, thermophilic hyphae, and ability to secrete serine proteases. The cardinal histological feature of ABPM is allergic (eosinophilic) mucin-harboring hyphae in the bronchi, for which the formation of extracellular DNA trap cell death (ETosis) of eosinophils induced by viable fungi is essential. Clinically, ABPM is characterized by peripheral blood eosinophilia, increased IgE levels in the serum, IgE and IgG antibodies specific for fungi, and characteristic radiographic findings; however, there are substantial differences in the clinical features of this disease between East and South Asian populations. Systemic corticosteroids and/or antifungal drugs effectively control acute diseases, but recurrences are quite common, and development of novel treatments are warranted to avoid adverse effects and emergence of drug-resistance due to prolonged treatment with corticosteroids and/or antifungal drugs.
References
1. Hinson KF, Moon AJ, Plummer NS. Bronchopulmonary aspergillosis; a review and a report of eight new cases. Thorax. 1952; 7:317–33.

2. Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013; 51:361–70.

3. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, Moss R. Denning DWABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013; 43:850–73.

4. Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012; 129:280–91.

5. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015; 70:270–7.

6. Agarwal R, Khan A, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A. An alternate method of classifying allergic bronchopulmonary aspergillosis based on high-attenuation mucus. PLoS One. 2010; 5:e15346.

7. Oguma T, Taniguchi M, Shimoda T, Kamei K, Matsuse H, Hebisawa A, Takayanagi N, Konno S, Fukunaga K, Harada K, Tanaka J, Tomomatsu K, Asano K. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol Int. 2018; 67:79–84.

9. Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: resolved and unresolved issues. Allergol Int. 2015; 64:321–31.

10. Portnoy JM, Williams PB, Barnes CS. Innate immune responses to fungal allergens. Curr Allergy Asthma Rep. 2016; 16:62.

12. Chamilos G, Ganguly D, Lande R, Gregorio J, Meller S, Goldman WE, Gilliet M, Kontoyiannis DP. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One. 2010; 5:e12955.

13. Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol. 2014; 40:30–48.

14. Oshikata C, Watanabe M, Saito A, Yasueda H, Akiyama K, Kamata Y, Tsurikisawa N. Allergic bronchopulmonary mycosis caused by Penicillium luteum. Med Mycol Case Rep. 2016; 15:9–11.

15. O'Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005; 5:4.
16. Araujo R, Rodrigues AG. Variability of germinative potential among pathogenic species of Aspergillus. J Clin Microbiol. 2004; 42:4335–7.

17. Imtiaj A, Jayasinghe C, Lee GW, Kim HY, Shim MJ, Rho HS, Lee HS, Hur H, Lee MW, Lee UY, Lee TS. Physicochemical requirement for the vegetative growth of schizophyllum commune collected from different ecological origins. Mycobiology. 2008; 36:34–9.

18. Oguma T, Asano K, Tomomatsu K, Kodama M, Fukunaga K, Shiomi T, Ohmori N, Ueda S, Takihara T, Shiraishi Y, Sayama K, Kagawa S, Natori Y, Lilly CM, Satoh K, Makimura K, Ishizaka A. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J Immunol. 2011; 187:999–1005.

19. Alp S, Arikan S. Investigation of extracellular elastase, acid proteinase and phospholipase activities as putative virulence factors in clinical isolates of Aspergillus species. J Basic Microbiol. 2008; 48:331–7.
20. Katzenstein AL, Liebow AA, Friedman PJ. Bronchocentric granulomatosis, mucoid impaction, and hypersensitivity reactions to fungi. Am Rev Respir Dis. 1975; 111:497–537.
21. Bosken CH, Myers JL, Greenberger PA, Katzenstein AL. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol. 1988; 12:216–22.

22. Hebisawa A, Tamura A, Kurashima A, Oobayashi C, Kawamata M, Maeda M, Saiki S, Komatsu H, Yoneda R. Pathologic reconsideration on allergic bronchopulmonary aspergillosis and mycosis. Nihon Kokyuki Gakkai Zasshi. 1998; 36:330–7.
23. Jelihovsky T. The structure of bronchial plugs in mucoid impaction, bronchocentric granulomatosis and asthma. Histopathology. 1983; 7:153–67.

24. deShazo RD, Swain RE. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995; 96:24–35.

25. Mukherji SK, Figueroa RE, Ginsberg LE, Zeifer BA, Marple BF, Alley JG, Cooper LL, Nemzek WR, Yousem DM, Jones KR, Kupferberg SB, Castillo M. Allergic fungal sinusitis: CT findings. Radiology. 1998; 207:417–22.

26. Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013; 121:2074–83.

27. Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep. 2016; 16:54.

28. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004; 303:1532–5.

29. Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, Vitkov L, van de Veerdonk FL, van den Berg TK, Roos D, Kuijpers TW. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol. 2016; 196:1272–83.
30. Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016; 137:258–67.

31. Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018; 141:571–585. e7.

32. Ueki S, Ohta N, Takeda M, Konno Y, Hirokawa M. Eosinophilic otitis media: the aftermath of eosinophil extracellular trap cell death. Curr Allergy Asthma Rep. 2017; 17:33.

33. Agarwal R, Dhooria S, Singh Sehgal I, Aggarwal AN, Garg M, Saikia B, Behera D, Chakrabarti A. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest. 2018; 153:656–64.

34. Behera D, Guleria R, Jindal SK, Chakrabarti A, Panigrahi D. Allergic bronchopulmonary aspergillosis: a retrospective study of 35 cases. Indian J Chest Dis Allied Sci. 1994; 36:173–9.
35. Chakrabarti A, Sethi S, Raman DS, Behera D. Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses. 2002; 45:295–9.

36. Ishiguro T, Takayanagi N, Uozumi R, Baba Y, Kawate E, Kobayashi Y, Takaku Y, Kagiyama N, Shimizu Y, Morita S, Sugita Y. Diagnostic criteria that can most accurately differentiate allergic bronchopulmonary mycosis from other eosinophilic lung diseases: a retrospective, single-center study. Respir Investig. 2016; 54:264–71.

37. Kim JH, Jin HJ, Nam YH, Hwang EK, Ye YM, Park HS. Clinical features of allergic bronchopulmonary aspergillosis in Korea. Allergy Asthma Immunol Res. 2012; 4:305–8.

38. Prasad R, Garg R, Sanjay , Shukla AD. Allergic bronchopulmonary aspergillosis: a review of 42 patients from a tertiary care center in India. Lung India. 2009; 26:38–40.

39. Tanimoto H, Fukutomi Y, Yasueda H, Takeuchi Y, Saito A, Watai K, Sekiya K, Tsuburai T, Asano K, Taniguchi M, Akiyama K. Molecular-based allergy diagnosis of allergic bronchopulmonary aspergillosis in Aspergillus fumigatus-sensitized Japanese patients. Clin Exp Allergy. 2015; 45:1790–800.

40. Ye F, Zhang NF, Zhong NS. Clinical and pathological analysis of allergic bronchopulmonary aspergillosis in China. Zhonghua Jie He He Hu Xi Za Zhi. 2009; 32:434–8.
41. Zhang M, Gao J. Clinical analysis of 77 patients with allergic bronchopulmonary Aspergillosis in Peking Union Medical College Hospital. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017; 39:352–7.
42. Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009; 13:936–44.
43. Shah A, Kunal S. A review of 42 asthmatic children with allergic bronchopulmonary aspergillosis. Asia Pac Allergy. 2017; 7:148–55.

44. Asano K. Innate immune systems: a clue in the pathogenesis of adult-onset, eosinophilic lung diseases. Respir Investig. 2017; 55:93.

45. Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981; 36:345–9.

46. Ishiguro T, Takayanagi N, Kagiyama N, Shimizu Y, Yanagisawa T, Sugita Y. Clinical characteristics of biopsy-proven allergic bronchopulmonary mycosis: variety in causative fungi and laboratory findings. Intern Med. 2014; 53:1407–11.

47. Greenberger PA, Patterson R. Allergic bronchopulmonary aspergillosis and the evaluation of the patient with asthma. J Allergy Clin Immunol. 1988; 81:646–50.

48. Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977; 86:405–14.

49. Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, Knutsen AP. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2014; 2:703–8.

50. Agarwal R, Aggarwal AN, Garg M, Saikia B, Chakrabarti A. Cut-off values of serum IgE (total and A. fumigatus -specific) and eosinophil count in differentiating allergic bronchopulmonary aspergillosis from asthma. Mycoses. 2014; 57:659–63.

51. Pashley CH, Fairs A, Morley JP, Tailor S, Agbetile J, Bafadhel M, Brightling CE, Wardlaw AJ. Routine processing procedures for isolating filamentous fungi from respiratory sputum samples may underestimate fungal prevalence. Med Mycol. 2012; 50:433–8.

52. Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: a review. Med Mycol. 2014; 52:782–97.

53. Agarwal R, Maskey D, Aggarwal AN, Saikia B, Garg M, Gupta D, Chakrabarti A. Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: a latent class analysis. PLoS One. 2013; 8:e61105.

54. Agarwal R, Aggarwal AN, Dhooria S, Singh Sehgal I, Garg M, Saikia B, Behera D, Chakrabarti A. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J. 2016; 47:490–8.

55. Agarwal R, Aggarwal AN, Sehgal IS, Dhooria S, Behera D, Chakrabarti A. Utility of IgE (total and Aspergillus fumigatus specific) in monitoring for response and exacerbations in allergic bronchopulmonary aspergillosis. Mycoses. 2016; 59:1–6.

56. Ishiguro T, Takayanagi N, Takaku Y, Kagiyama N, Shimizu Y, Yanagisawa T, Kawabata Y, Sugita Y. Allergic bronchopulmonary aspergillosis with repeated isolation of nontuberculous mycobacteria. Intern Med. 2013; 52:1721–6.

57. Agarwal R, Khan A, Aggarwal AN, Saikia B, Gupta D, Chakrabarti A. Role of inhaled corticosteroids in the management of serological allergic bronchopulmonary aspergillosis (ABPA). Intern Med. 2011; 50:855–60.

58. Moreira AS, Silva D, Ferreira AR, Delgado L. Antifungal treatment in allergic bronchopulmonary aspergillosis with and without cystic fibrosis: a systematic review. Clin Exp Allergy. 2014; 44:1210–27.

59. Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 63:e1–60.

60. Kohno S, Tamura K, Niki Y, Izumikawa K, Oka S, Ogawa K, Kadota J, Kamei K, Kanda Y, Kiuchi T, Shibuya K, Takakura S, Takata T, Takesue Y, Teruya K, Tokimatsu I, Fukuda T, Maesaki S, Makimura K, Mikamo H, Mitsutake K, Miyazaki Y, Mori M, Yasuoka A, Yano K, Yamanaka N, Yoshida M. Executive summary of Japanese domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016; 57:E117–63.

Fig. 1.
Histology of allergic (eosinophilic) mucin in the bronchi. Mucus plugs packed in the bronchi of surgically-resected lungs from a patient with allergic bronchopulmonary mycosis demonstrate a fir tree-like structure (A: H&E, x5), which contain many eosinophils, Charcot-Leyden crystals, and fibrin exudates (B: H&E, x40). (C) Hyphae of fungi are sparsely found in mucus plugs (Grocott staining, x40).
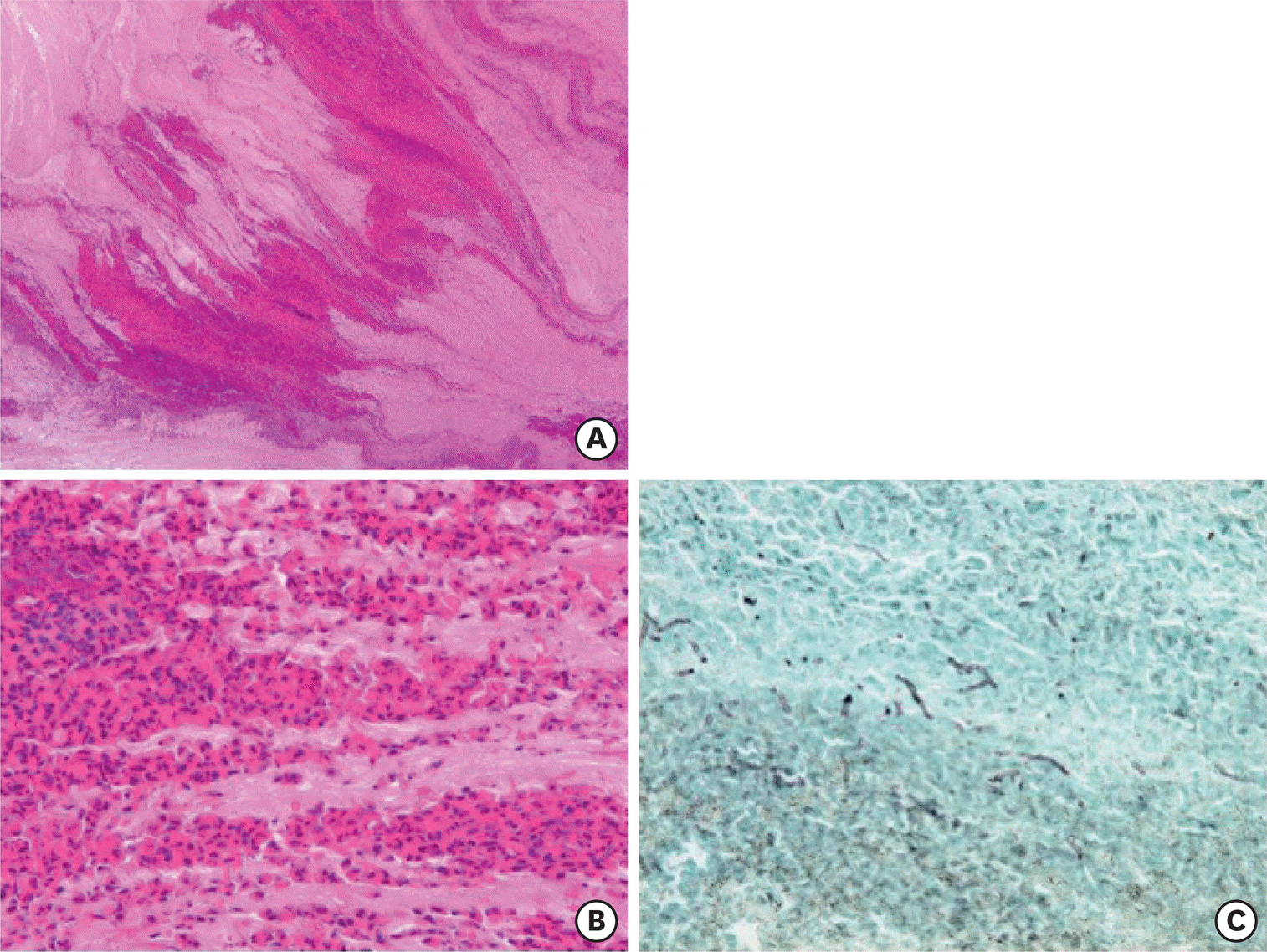
Table 1.
Clinical characteristics of allergic bronchopulmonary aspergillosis (ABPA) in East and South Asia
Table 2.
Comparison of diagnostic criteria




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download