Abstract
Hydatid cyst, a common disease in the world, is usually transmitted to humans through dog feces. Hydatid cyst is caused by Echinococcus granulosus. Diagnostic interventions for hydatid cyst include physical examination and chest x-ray tomography. Although the treatment options of hydatid cyst vary according to the clinical findings of the patients, the primary treatment may be considered as surgery. We herein reported the case of a child hospitalized due to pneumonia who developed anaphylaxis as a result of the rupture of a pulmonary hydatid cyst.
Hydatid cyst, an infection of echinococcus granulosus, is commonly encountered in our country. The disease is endemically seen in countries which deal large in agriculture and husbandry. A parasitic disease, it is mostly transmitted to humans through contamination of canis feces. The eggs in canis feces are discharged and ingested by human beings orally through contaminated water or foods. The embryos emerge from the eggs in the duodenum and then they are transferred mostly to the liver, and secondly to the lungs via the portal vein or lymphatic drainage respectively. In these organs, the embryos are transformed into the larval stage and thereby produce hydatidosis (hydatid cyst) [1].
The disease is endemic in Turkey, Mediterranean countries, South Africa, the Middle East, South America, and New Zealand. According to the records from the Ministry of Health, 51,500 diagnosed cases of hydatid cyst were reported by hospitals affiliated to the Ministry between 1965 to 1995. The prevalence of the disease is 50-400 per 100,000 people and the incidence is 3.4 per 100,000 persons in Turkey [2, 3].
In cases of hydatid cyst, the liver and lungs are involved most of the time, but rarely the bone, muscle, brain, kidney, and spleen are affected. The signs and symptoms depend on the size of the cyst, the organ involved and the interaction between the cyst and neighboring organs. It has been reported that some few cysts may stay asymptomatic and may spontaneously shrink with time [4].
The cyst has a high tendency to involve the lungs in children when compared to adults. In pulmonary hydatid cyst, cough, dyspnea, chest pain, flank pain, hemoptysis, and secondary pneumonia can occur due to compression of neighboring tissues by the cyst. Occasionally, the rupture of the cyst can result in expectoration of cystic fluid, empyema, and abscess formation rarely allergic reaction and anaphylaxis can occur. Anaphylaxis due to rupture of a hepatic hydatid cyst has often been reported however rupture it is very rare in a pulmonary hydatid cyst [1].
We herein reported the case of a child hospitalized due to pneumonia who developed anaphylaxis as a result of the rupture of a pulmonary hydatid cyst. Due to this rarely seen presentation, we wanted to emphasize the importance of hydatid cyst in the differential diagnosis of pneumonia in the event of similar radiologic and clinical findings in children living in endemic regions.
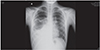
A 13-year-old boy without any known chronic illness was admitted to our outpatient policlinic with complaints of fatigue, fever, sputum expectoration, vomiting, loss of appetite, and acute attacks of choking cough especially at night. The complaints had not decreased over the last 20 days despite the patient using different types of antibiotics. His general physical appearance was pale and exhausted. He had subfebrile fever of 37.5℃ and blood pressure of 110/70 mmHg. Physical examination revealed bilaterally reduced pulmonary sounds at the mid and lower zones with bilateral crepitations which were heard more in the right lung. Abdominal examination was normal without hepatosplenomegaly. Laboratory findings showed leukocyte of 38,470/mm3(consisted of 92% neutrophil, 4.2% lymphocyte, 2.1% monocyte, and 1.5% eosinophil), hemoglobin of 14.3 g/dL, platelet of 437,000/µL and C-reactive protein of 120 mg/dL. Posteroanterior chest x-ray showed extensive infiltration in the mid and lower zones of the right lung with a round-shaped air-filled lesion (Fig. 1). Thoracic ultrasonography confirmed the presence of bronchogram in the lower zone of the right lung as a pneumonic consolidation. The possibility of abscess was excluded. Ampicillin/sulbactam in combination with amikasin was started. On the third day of treatment, the patient developed a confused state of mind together with tachypnea (60 times per minute), dyspnea, tachycardia (130 beats per minute), hypoxemia (oxygen saturation <90% under nasal oxygen flow at the rate of 6 L/min), and hypotension (75/40 mmHg). The patient was transferred to the pediatric intensive care unit. Considering possible shock status due to staphylococcal pneumonia, an aggressive intravenous fluid regimen and a change in antibiotic treatment to vancomycin and meropenem were started. Respiratory distress did not improve despite intensive application of inhaled β2 mimetic, inhaled corticosteroid and intravenous corticosteroid. The patient's immunity showed resistance to medical therapy and was clinically unstable. Since our hospital was lacking high-resolution computed tomography facility, the high resolution computed tomography (HRCT) examination could not be performed emergently.
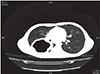
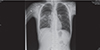
In this case, HRCT examination was performed after stabilization of the patient, it showed a lobulated, 9 cm × 7 cm × 7-cm lesion with cavitation in the superior segment of the lower lobe of the right lung indicating a ruptured hydatid cyst (Fig. 2). The diagnosis of anaphylaxis due to ruptured hydatid cyst was confirmed. Medical history also showed that the patient had experienced expectoration of cystic fluid together with an acute attack of severe cough just 30 minutes before the onset of shock status. A repeated hemogram test showed hemoglobin level of 12.4 g/dL, platelet of 276,000/µL, and leukocyte of 28,440/mm3. The peripheric blood film showed an eosinophilic ratio of 45%. The diagnosis of echinococcal infection was supported with positive indirect hemagglutination (IHA) and immunofluorescence antibody technique (IFAT) results of 1/640 and 1/1,000 respectively. The patient was given albendazole with an oral dose of 15 mg/kg/day twice a day. A cystectomy and capitonage operation was performed in the patient and a postoperative chest x-ray showed sufficient expansion of the lungs (Fig. 3). In the follow-up, abdominal ultrasonography and chest x-rays were normal without any other pathology. The patient was discharged with albendazole treatment and a further follow-up plan was made.
Hydatid cyst is endemic in our country and pulmonary hydatid cyst is commonly found among children in Turkey. Pulmonary cyst can lead to hemorrhages, congestion, bronchopneumonia, organized pneumonia, and obstructive pneumonia in neighboring lung tissue however it can also stay asymptomatic until reaching huge sizes and generally symptoms develop after traumatic or spontaneous rupture of the cysts. Secondary infections and rarely anaphylaxis can develop in cases of ruptured shrinking cysts or cysts draining into the bronchial system, respectively [5]. Similar to our case, Fanne et al. [6] reported a case of ruptured hydatid cyst leading to respiratory distress and anaphylactic shock in a 21-year-old female patient. Kayhan and Akgunes [7] reported a 93.3% incidence of cough and 6.6% of hemoptysis in their study. Pneumonia was considered as the initial diagnosis since the patient presented with fever, prolonged cough, and expectoration of sputum. We had assumed that thoracic ultrasonography would exclude the possibility of hydatid cyst suspected in chest x-ray. Sudden deterioration on follow-up suggested the possible diagnosis of staphylococcal pneumonia and pneumatocele. Persistant cough and respiratory distress together with hypotension and state of confusion were found as findings of anaphylaxis without skin involvement. In the literature, it is reported that 10% of anaphylaxis cases present without urticaria and angioedema [8]. Intravenous corticosteriod application after respiratory distress may also be a reason for the absence of skin findings. Shameem et al. [9] reported a case of ruptured hydatid cyst with pneumothorax and anaphylactic shock in a 22-year-old patient. Our case was 13 years old and without pneumothorax.
Chest x-ray is one of the most important basic tools for the diagnosis of pulmonary hydatid cyst. An air-filled cavitary lesion and lotus or meniscus signs can help in the diagnosis [10]. Use of ultrasonography is limited in the evaluation of pulmonary lesions. It can be helpful for lesions located near the chest wall [11]. Additionally, for exclusion of any other organ involvement, ultrasonography or magnetic resonance imaging can be performed [12, 13]. To avoid excess exposure to radiation in children, computed tomography (CT) is not used routinely as the first choice of diagnostic tool, although it gives detailed information about the cyst and surrounding organ involvement as well as being a very useful tool in excluding pulmonary abscess, tumors or mediastinal lymphadenopathies. In our case, due to the lesion having cystic appearance in the chest x-ray, we preferred thoracic ultrasonography which is easily done with no radiation and fast to apply in order to exclude abscess or cyst. Due to the sudden deterioration in the clinical condition of the patient, on the third day of hospitalization a thorax CT was considered for suspicion of staphylococcal pneumonia, but instead a ruptured hydatid cyst was finally diagnosed. Following CT results, additional serological tests were performed. IHA and IFAT for echinococcus were recorded as 1/64 and 1/1000 respectively.
The treatment modality for pulmonary hydatid cyst is surgery. Surgery targets the eradication of the parasite and closure of the remaining cavity [14]. Although there is not a consensus on the relevant parenchymal resection or parenchyma protective treatments, lobectomy, wedge resection, pericystectomy, and endocystectomy are some of the surgical treatment modalities used [15]. Here, cystectomy and capitonage were applied. Marashi et al. [16] reported a case of anaphylactic shock during an operation in a 42-year-old female patient and adviced attention to be given in this aspect. However the anaphylaxis developed preoperatively in our case. Thus, surgeons should be on alert for possible occurance of anaphylaxis and have necessary equipment ready for its managment.
Response of huge hydatid cyst to albendazole is limited but it can be used as an alternative to surgery in selected cases. Studies in the literature show that albendazole can be used in combination with surgery, especially in cases of inoperable cysts or in patients with high recurrence risk [12, 17]. In our patient, we used albendazole together with surgery.
In conclusion, early detailed radiological examinations should be done in cases with prolonged resistant pneumonia and suspected radiological lesions. Therefore, hydatid cyst should be considered in the differential diagnosis especially in endemic regions of Turkey and other regions around the world.
Figures and Tables
References
1. Tiryaki T, Senel E, Akbıyık F, Mambet E, Livanelioglu Z, Atayurt H. Kist hidatik hastalıklı çocuklarda on yıllık deneyimimiz. Türkiye Çocuk Hast Derg. 2008; 2:5–10.
2. Sayek I, Tirnaksiz MB, Dogan R. Cystic hydatid disease: current trends in diagnosis and management. Surg Today. 2004; 34:987–996.

4. Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996; 25:655–689.
5. Sakamoto T, Gutierrez C. Pulmonary complications of cystic echinococcosis in children in Uruguay. Pathol Int. 2005; 55:497–503.

6. Fanne RA, Khamaisi M, Mevorach D, Leitersdorf E, Berkman N, Laxer U, Maly B. Spontaneous rupture of lung echinococcal cyst causing anaphylactic shock and respiratory distress syndrome. Thorax. 2006; 61:550.

7. Kayhan S, Akgunes A. Histopatolojik Olarak Tanı Konulan Komplike Akciğer Kist Hidatik Olguları. Turkiye Parazitol Derg. 2011; 35:189–193.
8. Simons FE, Ardusso LR, Bilo MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY. World Allergy Organization. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011; 127:587–593. e1-22.

9. Shameem M, Akhtar J, Bhargava R, Ahmed Z, Khan NA, Baneen U. Ruptured pulmonary hydatid cyst with anaphylactic shock and pneumothorax. Respir Care. 2011; 56:863–865.

10. Celik M, Senol C, Keles M, Halezeroglu S, Urek S, Haciibrahimoglu G, Ersev AA, Arman B. Surgical treatment of pulmonary hydatid disease in children: report of 122 cases. J Pediatr Surg. 2000; 35:1710–1713.
12. Arroud M, Afifi MA, El Ghazi K, Nejjari C, Bouabdallah Y. Lung hydatic cysts in children: comparison study between giant and non-giant cysts. Pediatr Surg Int. 2009; 25:37–40.

13. Savas L, Onlen Y, Akcali C, Aslan B, Pourbagher A, Tunc T, Ozkoc G. Hydatid disease with atypical localization: 4 cases report. Scand J Infect Dis. 2004; 36:613–615.

14. Hasdiraz L, Oguzkaya F, Bilgin M. Is lobectomy necessary in the treatment of pulmonary hydatid cysts? ANZ J Surg. 2006; 76:488–490.

15. Halezeroglu S, Celik M, Uysal A, Senol C, Keles M, Arman B. Giant hydatid cysts of the lung. J Thorac Cardiovasc Surg. 1997; 113:712–717.

16. Marashi S, Hosseini VS, Saliminia A, Yaghooti A. Anaphylactic shock during pulmonary hydatid cyst surgery. Anesth Pain Med. 2014; 4:e16725.

17. Morris DL, Dykes PW, Marriner S, Bogan J, Burrows F, Skeene-Smith H, Clarkson MJ. Albendazole: objective evidence of response in human hydatid disease. JAMA. 1985; 253:2053–2057.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download