Abstract
Background
Henoch-Schönlein purpura (HSP) is the most common vasculitis in children, characterized by triad of symptoms; palpable purpura without thrombocytopenia, abdominal pain, and arthritis. Renal involvement often occur in children with HSP. No data on the renal involvement of children with HSP in Indonesia, especially West Java.
Methods
Retrospective study was conducted in children with HSP in Department of Child Health, Hasan Sadikin Hospital, from 2006 to 2011. Characteristics and clinical manifestations was reviewed from medical record. HSP was diagnosed by American College of Rheumatology 1990 criteria or European League Against Rheumatism/Pediatric Rheumatology International Trials Organization/Pediatric Rheumatology European Society 2008.
Results
There were 128 patients, consisting of 82 male (64.9%) and 46 female (35.1%) with ratio 1.8:1. Mean age was 7.9 ± 2.9 years old which range from 6 month to 15 years. Peak morbidity was between 5-10 years old. Prevalence of HSP in Hasan Sadikin Hospital tend to raise from 2.7/100,000 in 2008 to 5.2/100,000 in 2010. In most patients (71%) purpura was the first symptom. Seventy-one patients (44.5%) had arthritis and 89 patients (69.5%) had abdominal pain, while renal involvement was in 28 patients (21.8%). Gastrointestinal manifestations tend to manifest in patients less than 5 years old (p = 0.267), while renal involvement tend to manifest in age group 11-15 years old (p = 0.015) with odds ratio 3.1 (95% confidence interval, 1.2-8.1).
Henoch-Schönlein purpura (HSP) is the most common vasculitis in children, occurring in 8 to 20.4 per 100,000 children per year [1, 2]. HSP usually occurs in children aged between 2-10 years, with 50% of all cases occurred in children aged <5 years, mostly in children aged 4-6 years and occurs more frequently in male [1, 2]. Although it is generally a self limiting conditions, HSP can cause acute gastrointestinal manifestations (bleeding, intussusception), and renal manifestations with various incidence [3-8]. Age of onset is a suspected risk factors for renal manifestations of HSP [8].
Etiology of HSP is unknown, although a variety of antigens such as infections, vaccinations, drugs, foods and insect bites can trigger the onset of HSP [5]. The disease is characterized by deposits of immunoglobulin A (IgA) which contained immune complexes and complement components in small blood vessel walls [4]. In this study, we evaluated renal involvement in patients who diagnosed as HSP, who came to Division of Allergy-Immunology, Department of Child Health, Hasan Sadikin Hospital (Bandung, Indonesia) from 2006 to 2011.
This was a retrospective study, evaluated children who diagnosed with HSP, during October 2006-October 2011. Diagnosis of criteria was American College of Rheumatology 1990. After European League Against Rheumatism, Pediatric Rheumatology International Trials Organization, and Pediatric Rheumatology European Society criteria was published in 2008, later criteria was used (Table 1).
Data of patients who diagnosed as HSP, who came to Division of Allergy Immunology, was obtained from medical records, including age at diagnosis, sex, clinical symptoms, and laboratory profile. Clinical symptoms were recorded, such as purpura, abdominal pain, arthritis, presence of nephritis; also the possibility of precipitating factors that precede the occurrence of HSP on the history of infections, including upper respiratory infections, dental infections, skin infection, tuberculosis, and diarrhea that occur preceding history of HSP. Statistical analysis performed were chi-square and analysis of variance test.
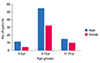
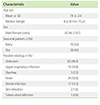
There were 128 children who diagnosed as HSP. Characteristics of patients were listed in Table 2. There were more male than female patients in all age groups as shown in Fig. 1. The incidence of HSP varies each month. HSP mainly occur in January-March (29.0%) and May-July (34.9%) as shown in Fig. 2. There was an increasing trends of HSP prevalence during 2008-2010 period. In 2008 the prevalence of HSP was 2.7/100,000 and increase to 5.2/100,000 in 2010 (Fig. 3).
Arthritis occured in 57 patients (44.5%), and arthralgia only was occured in 14 (11%). In 13 cases (10.2%) arthritis or arthralgia was the first manifestation of HSP.
Abdominal pain was complained in 89 from 128 patients (69.5%). In 35 cases (41.4%) there were positive result for occult blood in feses. Invagination/intussusseption was occured in three cases (2.3%), intestinal perforation and cholecystitis was occured in each one case (0.8%), which diagnosed from ultrasonography. Abdominal pain as first symptoms in HSP was occurred in 24 patients (18.8%). Gastrointestinal manifestation tended to occur in children less than 5 years old.
There were various degrees of renal abnormality in 28 children (21.9%). Mild renal involvement occured in eight cases (7.1%). Severe renal involvement occurred in 20 cases (17.9%). Most patients with renal involvement was above 5 years old (26 from 28 cases, 93%), especially in 11-15 years (p=0.022) (Table 5). The age group 11-15 years old had odds ratio (3.1; 95% confidence interval, 1.2-8.1) for renal involvement (Table 6).
The central nervous system involvement was occurred in two patients (1.6%) with seizure as clinical manifestation. Cholecystitis and myocarditis each was occured in one case (0.8%).
Laboratory profile was shown in Table 7. There were raised in blood sedimen rate in 20 of 32 patients (62.5%), thrombocytosis in 67 of 116 patients (57.8%), leucocytosis in 53 of 116 patients (45.7%), and anemia in 80 of 116 patients (31%).
In this study, there were 128 children who diagnosed with HSP at 5-year period. The mean of age was 7.9 ± 2.9 years, slightly older than the study Trapani et al. [7] to 6.1 ± 2.7 years and Aalberse et al. [2] with a mean age of 6 years when diagnosed, with a wider age range 0-18 years. The mean of age in this study need to evaluate, whether there was a delay in diagnosis. HSP occurred in the age range 6 months to 15 years, similar to other studies that show a wide age range in the HSP, but rarely at age <2 years, and in this study only one patient aged <2 years (0.8%). In other study, prevalence of patients <2 years was 5.3% [7]. At the age of <2 years, HSP has a generally mild clinical symptoms, limited to skin manifestations, and the presence of edema, joint involvement, but rare complication of abdominal nor nephritis and have a good prognosis. Comparison of boys and girls in this study was 1.8:1, similar to other studies that indicate a male predominance [2, 7, 8].
In addition to the increasing trend of prevalence, this study obtained a lower prevalence than other studies. In 2010 the prevalence of HSP is likely to increase to 5.2/100,000 from 2.7/100.000 in 2008. Gardner-Medwin et al. [1] and Aalberse et al. [2] reported incidence of 8 to 20.4 per 100,000 children per years.
The relationship between HSP with a history of infection has been described by various research [7]. In this study the most common possible infection was upper respiratory tract infection in 14.8%, 13.3% of dental infections and history of fever without other symptoms in 18.8%. Although the etiology is not known, but various pathogens have been suspected as the origin of HSP, most of which are β-hemolytic Streptococcus group A. The high dental infection that precedes the HSP requires special attention, because a study in Japan (2011) showed dental as a focus of infection in only 4.2% cases [9]. There is a history of gastrointestinal tract infection with symptoms of diarrhea, skin diseases and tuberculosis in 3, 2, and 1 cases; these were rare infection remains to be investigated. In the tuberculosis that preceded HSP, presumably immune complexes on the endothelial wall as a cause of vasculitis by the finding of mycobacteria antigen in vasculitis associated with tuberculosis [10]. This research has not shown the existence of other antigenic factors as a possible trigger HSP, such as immunization history, insect bites, or streptococcal antigen [5, 11].
There were purpuras in all of the study patients, which is the main diagnostic criteria. In HSP, purpura predominantly found in the lower limbs and buttocks, and if not found in the predilection area it is necessary to do a skin biopsy. In this study there were complaints of abdominal pain in 69.5% patients, slightly higher than the study by Trapani et al. [7] as much as 51% of patients and similar to research Peru et al. [8] as much as 66%.
Nephritis in various degrees in this study occured in 21.8% patients. This amount is still lower than Trapani et al. [7] and Peru et al. [8] studies with 54% and 30% of patients. The frequency of HSP nephritis in various studies varies from 15% to 62%. The risk of renal failure in patients with HSP nephritis child reaches about 20% in 20 years. Nephritis symptoms may include hematuria and proteinuria, but heavy proteinuria in the early diagnosis is a poor predictor of the risk of renal failure [7, 8, 12]. In this study, renal involvement were more prevalent in the age group 11-15 years, this group had a risk of renal involvement 3 times higher than the age group ≤10 years; different with Peru et al. [8] report, there is no difference in the renal involvement according to age.
Abdominal pain is a frequent complaint of patients coming to hospital and complaints are sometimes preceded by purpura. Abdominal complication that often occurs is intussusception or invagination that require immediate operative treatment, although some reports have shown an improvement with conservative treatment [7, 8, 12].
Arthritis in this study occurred in 44.5% of patients, lower than the study of Trapani et al. [7] and Peru et al. [8] respectively 74% and 56%. Nevertheless, study of Trapani et al. [7] shows that 74% of patients, consisting of 61% of arthritis and others are arthralgia. This can occur because of arthritis and arthralgia tend to be neglected in children, because not all children are able to tell the complaints.
There are increased of erythrocyte sedimentation rate in 20 of 32 patients (62.5%), thrombocytosis in 67 of 116 patients (57.8%), leukocytosis in 53 of 116 (45.7%), and anemia in 80 of 116 patients (31%) this similar with study in Italy and Turkey [8, 9].
In conclusion, renal involvement occur more in children of age group 11-15 years old, while gastrointestinal manifestations tend to occur in children less than 5 years old. HSP patients in this study show a similar characteristcs and clinical profile with most of other study. There were increasing trends of HSP over a 5-year period.
Figures and Tables
Table 1
Diagnosis using ACR (1990) or EULAR/PRINTO/PRES (2008)

ACR, American College of Rheumatology; EULAR/PRINTO/PRES, European League Against Rheumatism/Pediatric Rheumatology International Trials Organization/Pediatric Rheumatology European Society.
Adapted from Ozen S, et al. Ann Rheum Dis 2010;69:798-806 [6].
Table 3
Characteristics of Henoch-Schönlein purpura patients (n = 128)

*Mild renal involvement: microscopic hematuria and or proteinuria without nephritis; severe renal involvement: proteinuria >40 mg/m2 per hour and or acute nephritic syndrome (macroscopic/microscopic hematuria with at least 2 of 3: oliguria, hypertension, and decrease in renal functions); renal insufficiency: creatinine >125% above normal limit.
References
1. Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002; 360:1197–1202.
2. Aalberse J, Dolman K, Ramnath G, Pereira RR, Davin JC. Henoch Schonlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis. 2007; 66:1648–1650.

3. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009; 80:697–704.
4. Lau KK, Suzuki H, Novak J, Wyatt RJ. Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2010; 25:19–26.

5. Sinclair P. Henoch-schönlein purpura: a review. Curr Allergy Clin Immunol. 2010; 23:116–120.
6. Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, Rigante D, Cantarini L, Hilario MO, Silva CA, Alegria M, Norambuena X, Belot A, Berkun Y, Estrella AI, Olivieri AN, Alpigiani MG, Rumba I, Sztajnbok F, Tambic-Bukovac L, Breda L, Al-Mayouf S, Mihaylova D, Chasnyk V, Sengler C, Klein-Gitelman M, Djeddi D, Nuno L, Pruunsild C, Brunner J, Kondi A, Pagava K, Pederzoli S, Martini A, Ruperto N. Paediatric Rheumatology International Trials Organisation (PRINTO). EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008 Part II: Final classification criteria. Ann Rheum Dis. 2010; 69:798–806.
7. Trapani S, Micheli A, Grisolia F, Resti M, Chiappini E, Falcini F, De Martino M. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005; 35:143–153.

8. Peru H, Soylemezoglu O, Bakkaloglu SA, Elmas S, Bozkaya D, Elmaci AM, Kara F, Buyan N, Hasanoglu E. Henoch Schonlein purpura in childhood: clinical analysis of 254 cases over a 3-year period. Clin Rheumatol. 2008; 27:1087–1092.

9. Nakaseko H, Uemura O, Nagai T, Yamakawa S, Hibi Y, Yamasaki Y, Yamamoto M. High prevalence of sinusitis in children with henoch-schönlein purpura. Int J Pediatr. 2011; 2011:562638.

10. Islek I, Muslu A, Totan M, Gok F, Sanic A. Henoch-Schonlein purpura and pulmonary tuberculosis. Pediatr Int. 2002; 44:545–546.

12. Davin JC. Henoch-Schonlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol. 2011; 6:679–689.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download