Abstract
The increase in the incidence of food allergy is a growing problem for the western world. This review will focus on the findings from several macromolecular epithelial transport experiments and drug permeability studies to provide a recent comprehension of food allergen intestinal epithelial cell transport and the allergen-epithelial relationship. Specifically, this review will aim to answer whether allergens can permeate the intestinal barrier directly via intestinal epithelial cells, and whether this mode of transport affects downstream immune reactions. By improving our understanding of the interactions which take place during exposure of food allergens with the intestinal epithelium, we can begin to understand whether the epithelial barrier plays a major role in the allergic sensitization process rather than simply restricting the entry of allergens to the underlying lamina propria.
Today, it's not uncommon to hear of allergy to common foods such as egg, milk, seafood, many fruits, tree nuts and in particular peanuts. Peanuts in particular are the highest contributor of anaphylactic deaths after ingestion of a food allergen [1]. Only trace amounts of peanut protein can trigger an allergic response [2]. It is for this reason that 'nut-free' schools, kindergartens and childcare facilities is nowadays common practice. The food allergy epidemic continues to lead the allergy and immunology zeitgeist, and will remain at the forefront of allergist investigation until a definitive understanding of food allergy cause and manifestation is achieved.
Food allergy is a growing problem for the western world. Recently, an Australian study involving a small population of children; referred to a specialist allergy clinic within the Australian Capital Territory, revealed that the incidence of peanut sensitivity in children within Australia has more than doubled since 1995 [3]. A 2009 study-conducted in the United Kingdom by Venter et al. [4], compared the prevalence of peanut allergy for three different cohorts of children A, B, and C whom were either born in 1989, 1994-1996 and 2001-2002, respectively. Peak peanut sensitisation was observed in cohort B at 3.3%, an increase to that observed in 1989 (1.3%). Interestingly, cohort C observed statistically non-significant decrease at 2.0%. Similarly, another study revealed that the prevalence of peanut allergy has almost doubled from 0.24 per 1,000 patients in 2001 to 0.51 per 1,000 patients in 2005 as observed by an increase in diagnoses by general practitioners [5]. Other studies have also demonstrated similar findings [6, 7]. This rising trend highlights the importance of global therapeutic intervention and research targeted at combating the rising prevalence of peanut allergy.
Currently, the best means of managing the condition is strict avoidance. Novel therapies, such as oral immunotherapy; involving the administration of small amounts of allergen in a strictly controlled clinical setting is showing potential of being an effective treatment [8]. Despite this, peanut allergy has an inherent risk of fatal anaphylaxis [1], and is often caused by trace amounts of peanut [2]. Thus, not only should oral immunotherapy be approached with caution, the therapy may only be suitable for individuals with a mild response after allergen stimulation.
The growing incidence of peanut allergy throughout westernised populations; and the limited available therapeutic intervention, purports the development of additional effective treatments to mediate the condition. In addition, further study into the mechanism of peanut allergy should be of global priority, to prevent greater economic burden in the future. Therefore, the aim of this review will focus on a relatively unexplored aspect of the allergen sensitization process-the initial passage through the intestinal epithelium. The findings from several macromolecular epithelial transport experiments and drug permeability studies have been collated to provide a recent comprehension of food allergen intestinal epithelial cell (IEC) transport. IEC allergen transport is relatively unaddressed in the literature. Thus, expanding our knowledge in this field will enhance our understanding of the allergen sensitization process. To address this, our review will briefly introduce the allergenic characteristics of the major allergenic food peanut, and the process by which an individual becomes sensitized to peanuts: from allergen to allergy. From here, the review will explore the various pathways that allergens may cross the intestinal mucosa via IEC. Specifically, this review will aim to answer whether peanut allergens are likely to permeate the intestinal barrier via IEC.
Certain characteristics of peanut allergens increase their allergenicity. These include the following: (1) Relative abundance. There are 11 peanut allergens described to date, namely Ara h 1 through to Ara h 11; (2) Thermal [9] and proteolysis [10] stability to heat treatment and digestion, respectively; (3) Numerous IgE binding sites [11-13], which are also often internalised. Thus, unreachable by various digestive enzymes [14].
Additionally, Ara h 2 is an effective inhibitor of trypsin [15]. More recently, Caco-2/TC7 cells stimulated with purified native Ara h 2 demonstrated a pro-inflammatory response, with up-regulated nitric oxide synthase (nos2a), interleukin (il)-6, and il-33 compared to proportional levels of lipopolysaccharide (LPS) [16]. Similarly, the epithelium-associated cytokine IL-33 was observed to play a role in instigating an allergic response to peanut [17], demonstrating not only the intrinsic interactions between allergens and the intestinal epithelium, but their capacity to interact and play a role in the downstream immune response to dietary allergens through their association with the intestinal epithelium.
Normally, allergen sensitization occurs as follows: antigens are selectively sampled by intestinal M cells (Fig. 1, ①), which are modified epithelial cells specially designed for the endocytosis of luminal antigens [18]. From here, the antigens are displayed to antigen presenting cells, such as macrophages, where they are degraded and incorporated into an antigen presenting protein complex termed the major histocompatability complex (MHC) or human leukocyte antigen (HLA). Specifically, MHC class II or HLA subgroup D, respectively, are specific for allergen presentation. From here, down stream T and B cell activation will occur, which will ultimately result in the production of chemical mediators to elicit an immune response. Interestingly, Dreskin et al. [19], observed that HLA class II may not play a major role in the development of peanut allergy when comparing sets of siblings, which comprised of an allergic and non-allergic individual with identical HLA. Interestingly, this study also observed the occurrence of a relatively rare allele DRB1*0803, which may prove to be a risk factor for the predisposition to peanut allergy. However, further research is necessary to investigate this correlation. What this study does suggest however, is that individual MHC differences does not determine whether an individual will obtain peanut allergy. However, environmental influences, antigen processing and epitope presentation may contribute significantly to the downstream sensitisation cascade. Here, allergen properties may impact on the extent of antigen processing prior to incorporation into MHC II. Therefore, mechanisms which can modify the allergen prior to MHC II incorporation may provide a means for understanding the molecular etiology of some food allergies.
The allergen sensitization begins with the transport across the intestinal epithelium. Transport of luminal antigens is typically via M cells but IECs also have the capacity to transport luminal antigens across the epithelium (Fig. 1, ② to ③), but to a different capacity to M cells. Peanut allergens have been detected in breast milk [20] suggesting that intact allergens can cross the intestinal epithelium and enter the circulatory system without processing. Whether their passage is via IEC or M cells is explored below.
Antigen sampling by M cells is selective. Typically, this is the only means by which luminal antigens and macromolecules can cross the intestinal epithelia and are subject to processing by the gut immune system. Antigens which are often insoluble and/or aggregated are endocytosed only via M cells [21]. Here, antigens encounter less protective mechanisms such as mucous and microvilli to be transported easier. Antigens absorbed by M cells are transported directly to the underlying- immune cell rich- lamina propria, with little to no antigen alteration before presentation to a professional antigen presenting cell.
Despite this, some macromolecules have shown the ability to cross the intestinal epithelium without M cell passage, including allergens from milk [22], brazil nuts [23], and egg [24], just to name a few. This can occur transcellularly, where antigens are absorbed directly through epithelial cells or paracellularly, where antigens pass through the tight junctions joining IEC (Fig. 2, a). For an in depth explanation of all modes of epithelial transport, Tukker [25] has reviewed the topic in greater detail, which is beyond the scope of this review.
IECs in vivo and in vitro express many elements involved in antigen processing. This indicates a role for IEC in antigen presentation, independent of M cell involvement. HLA-DR was shown to be expressed in fetal intestinal epithelium from 18 weeks gestation, specifically at villi tips. This same study identified interferon gamma (IFN-γ) as the stimulant for HLA-DR expression by HT29 IEC in vitro [26]. IFN-γ has also shown to induce the expression of MHC class II in another IEC line Caco-2, further suggesting the role of IFN-γ secreting T-lymphocytes as the stimuli for MHC-II expression by IEC [27]. More recently, MoS13 IECs treated with IFN-γ were able to stimulate proliferation of CD4+ intestinal epithelial lymphocytes after antigen exposure; though not to the capacity of professional antigen presenting cells [28]. Despite this, levels of IFN-γ secreted by the CD4+ intestinal epithelial lymphocytes was significantly higher when in the presence of IEC compared to professional antigen presenting cells, where the increase in IFN-γ secretion was unique to intestinal epithelial CD4+ lymphocytes and not those found elsewhere in the body [28]. In addition to MHC-II expression and IFN-γ secretion by IEC, many additional antigen processing components have been identified in the IEC line HT29, which were comparable to those expressed in human small intestinal biopsy tissue [29]. Other studies have demonstrated similar results regarding IEC capability to secrete exosomes with antigen presenting components, with the potential capacity for antigen presentation to downstream immune cells [29-31], given that IEC have the capacity to absorb and process antigens. It is of particular interest to summarize any food allergens that have been observed to cross IEC, either paracellularly or transcellularly, independent of M cells.
Paracellular transport is directly related to the integrity of the intestinal epithelium and is controlled primarily by the tight junction (Fig. 2, a). Although transport via this way may not involve antigen processing by the IEC as such, IEC transport may influence the quantity of allergen passing through to the lamina propria. The tight junctions are located on the apicolateral cell membrane, and attach laterally to neighbouring cells. Thus, tight junctions are directly involved in the permeability and polarity of the intestinal epithelium, as their main function is to exclude luminal contents. Thus, any antigen that can cross here will be directly exposed to underlying immune cells in the lamina propria, effectively triggering the allergenic cascade, in an uncontrolled manner.
To explore the role that tight junctions play in disorders that alter intestinal permeability, including food allergy, we must first gain a brief understanding about the tight junction structure (Fig. 3).
The tight junction is comprised of two main units. Transmembrane protein complexes which span the paracellular space, and intracellular protein complexes which bind the structural elements of the cytoskeleton; such as actin. The transmembrane complexes are comprised of occludin [32], junctional adhesion molecule (JAM) [33] and members of the claudin family, which differ with tissue type [34]. Those proteins which are involved in the joining of the transmembrane proteins to the cytoskeleton, include zonula-occludins (ZO)-1 [35, 36], ZO-2 [36, 37], and ZO-3 [38], just to name a few. There are also numerous other proteins, which have been observed to localise to the tight junction, and are involved in cytoskeletal adhesion, but will not be explored in this review. They are however, adequately descried in several recent reviews by Niessen [39] and Gonzalez-Mariscal et al. [40].
The malfunctioning of tight junctions is associated with many diseases, including cancer, jaundice, edema, and colitis [41-44]. The role that the tight junction plays in these conditions is described in a review by Sawada et al. [45]. The function that the tight junction plays in the physiology of food allergy is less defined. Additionally, allergen paracellular IEC transport via tight junctions is scarcely addressed in the literature.
Experimental evidence for allergen transport across the intestinal mucosa is generally limited. However, a 2004 study by Chambers et al. [46], investigated the epithelial transport of peanut protein in BALB/c mice. This group observed both soluble protein and protein bodies in the cytoplasm of M cells within intestinal Peyer's patches. Interestingly, peanut bodies were also observed in the paracellular space between epithelial cells surrounding Peyer's patches but not in epithelial cells that were not adjacent to Peyer's patches [46]. This indicates the potential for peanut allergen paracellular passage, but also illustrates that peanut allergen IEC passage may be restricted to M cells only. Thus, more research is required to properly understand the transport of peanut allergens across the human intestinal mucosa.
The certain qualities of allergens that make them particularly more allergenic, for example heat resistance and digestion resistance, is reiterated with other potentially destructive qualities such as specific protease activity aimed at epithelial destruction. For example, although not a food allergen per se, the major house dust mite allergen Der p 1 effectively compromises the integrity of airway epithelium via tight junction destruction, resulting in the cleavage of occludin and increased paracellular permeability through a proteolytic mechanism [47]. This not only illustrates the invasive potential of successful allergens, such as those from dust mites, but also highlights the importance of the tight junctions in allergic disease.
Trancellular transport, or the movement of materials through epithelial cells, occurs either by simple diffusion (a), diffusion mediated by carrier proteins (b) or vesicular transport either by trancytosis (c), endocytosis (d) or active transport (e). For simple diffusion based transcellular transport, a compound is limited by its lipophilicity, solubility, concentration and absorption area. This could help to explain when the epithelial transport of the egg allergen ovomucoid was studied in Caco-2 cells, they were pre-treated with varying concentrations of food grade surfactants; the added surfactants effectively increased the permeability of the egg allergen when surfactant levels were < 50 µg/well or 25 µg/mL [24].
For more hydrophilic and poorly absorbed materials, carrier mediated transport is typically the main route. Carrier mediated transport can occur via diffusion, where, with the help of membrane bound carrier proteins, luminal particulates can be transported down its concentration gradient. Alternatively, this process can utilise a source of energy such as the conversion of adenosine triphosphate (ATP) to adenosine diphosphate to drive a protein pump, such as the sodium/potassium ATPase pump. This process can operate against a concentration gradient and is typically used for materials in very low external concentrations.
Endocytosis and transcytosis are two additional transport methods where external materials are engulfed by the cell membrane and enclosed and transported within vesicles within the cell. Generally, when analysing the transport method for various materials using cellular based models (i.e., Caco-2 monolayers), the transepithelial resistance (TEER) is used to address the integrity of the monolayer, where a change in TEER denotes alteration to tight junctions and thus relates to paracellular transport. Studies that exclude paracellular modes and thus accept transcellular modes, usually do so based on TEER and other markers for paracellular permeability. For example, purified wheat allergens ω5-gliadin and lipid transfer protein (LTP) were able to cross Caco-2 monolayers and detected by enzyme-linked immunosorbent assay (ELISA). Here, Caco-2 monolayer integrity was not compromised as no increase in permeability was observed via TEER or dextran flux. Also interestingly, digestion of the wheat allergens appeared to enhance their transcellular transport capacity [48], indicating the preferable uptake of digested allergens compared to whole allergens. It is likely that digestion may have rendered the allergen more soluble, allowing easier IEC absorption. Another study by Moreno et al. [23], reports the transcellular transport of purified 2s albumins Ber e 1 and Ses i 1 within Caco-2 monolayers, both of which are major brazil and white sesame seed allergens, respectively. Here, both Ber e 1 and Ses i 1 did not appear to affect permeability as observed with no change to allergen absorption rate, TEER or phenol red absorption. Considering this, we can assume that the peanut 2S albumins, Ara h 2, Ara h 6, and Ara h 7 [49] may have the potential to cross the epithelial barrier by similar means, due to conserved structural conformations. Recently, purified Ara h 2 has been observed to be endocytosed by HT-29 cells and targeted for lysosomal degradation after endosomal fusion, where the ubiquitin E3 ligase A20 is involved in its lysosomal degradation [50]. Also, the transport of the egg allergen ovalbumin is subject to its phosphorylation state, where it was found that apical to basal transport of dephosphorylated ovalbumin is reduced compared to its phosphorylated form, a result that is averted with the protease inhibitor leupeptin, suggesting lysosomal degradation during IEC transport [51]. Both of the above studies highlight the added importance of IEC digestion survival as an added element of allergen potency. Additionally of interest, the major soybean allergen, Gly m 1, has been shown to be endocytosed by IPEC-J2 cells in vitro [52].
As discussed earlier, protein solubility and aggregation can dictate the method of mucosal transport an allergen may take to cross the intestinal mucosa. While insoluble aggregated antigens are restricted to M cell transport, the more soluble non-aggregated antigens can potentially cross via normal IEC. This passage of soluble antigens through IEC is thought to be critical in the induction of allergic disease, at least for milk allergens [22]. Given this, it is important to note that allergens from roasted peanuts are less soluble than raw peanuts. However, insolubility can be ameliorated with amylase treatment or by a pH of 2 [9]. Additionally, Ara h 3, also upon heat treatment, formed insoluble aggregates, which were completely degraded during pepsin digestion, and thus incapable of significantly binding IgE [53]. Therefore, under normal digestive conditions, Ara h 3 does not appear to be a major contributor to sensitization to peanuts. However, in individuals with decreased digestive capacity, such as infants, Ara h 3 may reach the intestinal lumen, in its insoluble form aggregates, and may expose the immune system via M cell infiltration.
The type of heat treatment was also observed to affect the aggregation of Ara h 1. Where, boiling and roasting affected the major peanut allergens' aggregate structure, with the roasted aggregate of Ara h 1 retaining higher IgE reactivity compared to boiled Ara h 1 [54]. Similarly, Ara h 2 was also observed to aggregate after heat treatment [16]. Stronger immune eliciting capacity was confirmed in brown Norway rats for roasted peanuts compared to boiled peanuts. Interestingly, whether the peanuts were boiled or roasted did not effect the sensitization capacity of peanuts [55]. Also, recombinant Ara h 2 was more IgE reactive when heated compared to untreated, however negligible differences were observed between natural, untreated and heated Ara h 2 [16].
Given all this, it is likely that peanut allergen aggregates, made insoluble after heat treatment, do not have the capacity to cross the intestinal mucosa via IEC but only via M cells. Future research into this conjecture would be useful in the further understanding of the sensitization mechanism of peanut allergy.
Allergen transport across the intestinal mucosa is largely unreported in the literature. It is highly likely that allergen transport, gut permeability and tolerance induction are intrinsically linked in the development of food allergy.
Firstly, we have observed that allergen can cross the intestinal mucosa via IEC. Certain allergens such as the house dust mite Der p 1 has protease activity that can disrupt tight junctions and increase paracellular permeability. In vivo studies have shown that peanut protein bodies have been observed in the paracellular space of epithelial cells adjacent to M cells, but not in those further away from the Peyer's patches. On the other hand, in vitro studies have shown that soybean Gly m 1, can be endocytosed by IPEC-J2 cells [52] and purified Ara h 2 can be endocytosed by HT-29 cells and additionally targeted for lysosomal degradation after endosomal fusion [50]. In vitro studies have additionally shown the IEC transcellular translocation of the wheat allergens ω5 gliadin and lipid transfer protein [48], and the brazil nut and white sesame allergens Ber e 1 and Ses i 1 [23], respectively, all of which highlight the IEC translocating capacity of food allergens.
The absorption of food allergens from the gut lumen, without M cell passage, is not thoroughly explored. Additional means of absorption may well explain why some allergens are more potent allergens than others, like that of house dust mite, and why some food allergies are persistent through to adulthood. More data is required on the permeability and absorption of food allergens for a verdict to be made regarding whether mucosal permeability may increase an allergen's potency. Also, if more understanding is gained on additional modes of transport for specific food allergens, like Ber e 1 and Ses i 1 studies mentioned above, then this provides additional means for therapeutic targeting regarding specific allergies to foods. Currently, there is lack of data on the transport capabilities of many food allergens and their route of exposure to the mucosal immune system. This highlights the importance of further investigation into the absorption of food allergens across the intestinal mucosa.
Figures and Tables
Fig. 1
Antigen intestinal epithelial transport. Conventional antigen sampling of the intestinal luminal contents occurs via specialized epithelial cells termed M cells. Here, antigens are randomly selected from luminal contents ①. Those antigens which are insoluble and/or have a tendency to aggregate are restricted to crossing the mucosal barrier by this mechanism. Digested peanut allergens aggregate in neutral conditions, and are therefore likely restricted to mucosal passage via M cells only. Therefore, individuals who have a normal digestion capacity (years 2+) are more likely to have peanut allergens absorbed by this mechanism. Despite this, soluble and non-aggregated antigens have the capacity to cross the mucosal barrier through intestinal epithelial cells (IECs). This means that antigens in non-aggregated or more soluble states, such as undigested peanut allergens (peanut ingested by an individual with decreased digestion capacity or a 'leaky gut'; such as an infant) have an additional pathway for those allergens to cross the mucosal barrier and allow exposure to underlying immune cells. IEC passage may either be by parracellular transport ② or transcellular transport ③. Additionally, antigens may be processed and expressed on major histocompatability complex (MHC) II by IEC ④.
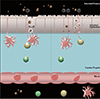
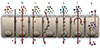
Fig. 2
The transport mechanisms of materials through epithelium. Paracellular transport (a), transcellular transport by diffusion (b), facilitated diffusion (c), transcytosis (d), endocytosis (e), active transport (f), and finally, efflux (g). Paracellular transport, is related to tight junction integrity, while transport mechanisms (b) to (f) are all loosely described as transcellular transport mechanisms. Efflux (g) will not be discussed in this review.
ACKNOWLEDGEMENTS
This work was supported by School of Life and Environmental Sciences and Molecular and Medical Research (MMR) Strategic Research Centre (SRC).
References
1. Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007; 119:1016–1018.

2. Hourihane JO'B, Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol. 1997; 100:596–600.

3. Mullins RJ, Dear KB, Tang ML. Characteristics of childhood peanut allergy in the Australian Capital Territory, 1995 to 2007. J Allergy Clin Immunol. 2009; 123:689–693.

4. Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, Higgins B, Dean T. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010; 65:103–108.

5. Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011; 127:623–630.e1.

6. Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002; 110:784–789.

7. Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003; 112:1203–1207.
8. Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009; 124:286–291.e6.

9. Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol. 2005; 136:16–22.

10. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996; 14:1269–1273.

11. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997; 245:334–339.

12. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm RM, West CM, Bannon GA. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997; 342:244–253.
13. Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, Bannon GA. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest. 1999; 103:535–542.

14. Maleki SJ, Kopper RA, Shin DS, Park CW, Compadre CM, Sampson H, Burks AW, Bannon GA. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J Immunol. 2000; 164:5844–5849.

15. Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung SY, Landry SJ. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003; 112:190–195.

16. Starkl P, Krishnamurthy D, Szalai K, Felix F, Lukschal A, Oberthuer D, Sampson HA, Swoboda I, Betzel C, Untersmayr E, Jensen-Jarolim E. Heating affects structure, enterocyte adsorption and signalling, as well as immunogenicity of the peanut allergen ara h 2. Open Allergy J. 2011; 4:24–34.

17. Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013; 131:187–200.e8.

18. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000; 16:301–332.

19. Dreskin SC, Tripputi MT, Aubrey MT, Mustafa SS, Atkins D, Leo HL, Song B, Schlichting D, Talwar H, Wang Q, Freed BM. Peanut-allergic subjects and their peanut-tolerant siblings have large differences in peanut-specific IgG that are independent of HLA class II. Clin Immunol. 2010; 137:366–373.

20. Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA. 2001; 285:1746–1748.

21. So AL, Small G, Sperber K, Becker K, Oei E, Tyorkin M, Mayer L. Factors affecting antigen uptake by human intestinal epithelial cell lines. Dig Dis Sci. 2000; 45:1130–1137.
22. Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, Jensen-Jarolim E, Mayer L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008; 63:882–890.

23. Moreno FJ, Rubio LA, Olano A, Clemente A. Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers. J Agric Food Chem. 2006; 54:8631–8639.

24. Mine Y, Zhang JW. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int Arch Allergy Immunol. 2003; 130:135–142.

25. Tukker JJ. Characterization of transport over epithelial barriers. In : Lehr CM, editor. Cell culture models of biological barriers: in vitro test systems for drug absorption and delivery. London: Taylor & Francis;2002. p. 52–61.
26. MacDonald TT, Weinel A, Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut. 1988; 29:1342–1348.

27. Lopes LM, Hughson E, Anstee Q, O'Neil D, Katz DR, Chain BM. Vectorial function of major histocompatibility complex class II in a human intestinal cell line. Immunology. 1999; 98:16–26.

28. Hatano R, Yamada K, Iwamoto T, Maeda N, Emoto T, Shimizu M, Totsuka M. Antigen presentation by small intestinal epithelial cells uniquely enhances IFN-γ secretion from CD4+ intestinal intraepithelial lymphocytes. Biochem Biophys Res Commun. 2013; 435:592–596.

29. Lin XP, Almqvist N, Telemo E. Human small intestinal epithelial cells constitutively express the key elements for antigen processing and the production of exosomes. Blood Cells Mol Dis. 2005; 35:122–128.

30. van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001; 121:337–349.

31. Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007; 132:1866–1876.

32. Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005; 57:883–917.

33. Severson EA, Parkos CA. Structure and function of JAM proteins. In : Ley K, editor. Adhesion molecules: function and inhibition. Basel: Birkhauser;2007. p. 271–288.
34. Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008; 1778:631–645.

35. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998; 273:29745–29753.

36. Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006; 126:741–754.

37. Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999; 274:5981–5986.
38. Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998; 141:199–208.

39. Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007; 127:2525–2532.

40. Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003; 81:1–44.
41. Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004; 198:748–757.
42. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007; 22:E4.

43. Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999; 116:301–309.

44. Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003; 22:2021–2033.

45. Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003; 36:147–156.

46. Chambers SJ, Wickham MS, Regoli M, Bertelli E, Gunning PA, Nicoletti C. Rapid in vivo transport of proteins from digested allergen across pre-sensitized gut. Biochem Biophys Res Commun. 2004; 325:1258–1263.

47. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999; 104:123–133.

48. Bodinier M, Legoux MA, Pineau F, Triballeau S, Segain JP, Brossard C, Denery-Papini S. Intestinal translocation capabilities of wheat allergens using the Caco-2 cell line. J Agric Food Chem. 2007; 55:4576–4583.

49. Moreno FJ, Clemente A. 2S albumin storage proteins: what makes them food allergens? Open Biochem J. 2008; 2:16–28.

50. Song CH, Liu ZQ, Huang S, Zheng PY, Yang PC. Probiotics promote endocytic allergen degradation in gut epithelial cells. Biochem Biophys Res Commun. 2012; 426:135–140.

51. Matsubara T, Akiyama Y, Oshima K, Okajima T, Nadano D, Matsuda T. Dephosphorylation reduces passage of ovalbumin antigen through intestinal epithelial Caco-2 cell monolayers. J Biochem. 2013; 153:347–354.

52. Sewekow E, Bimczok D, Kahne T, Faber-Zuschratter H, Kessler LC, Seidel-Morgenstern A, Rothkotter HJ. The major soyabean allergen P34 resists proteolysis in vitro and is transported through intestinal epithelial cells by a caveolae-mediated mechanism. Br J Nutr. 2012; 108:1603–1611.
53. van Boxtel EL, van den Broek LA, Koppelman SJ, Gruppen H. Legumin allergens from peanuts and soybeans: effects of denaturation and aggregation on allergenicity. Mol Nutr Food Res. 2008; 52:674–682.

54. Blanc F, Vissers YM, Adel-Patient K, Rigby NM, Mackie AR, Gunning AP, Wellner NK, Skov PS, Przybylski-Nicaise L, Ballmer-Weber B, Zuidmeer-Jongejan L, Szepfalusi Z, Ruinemans-Koerts J, Jansen AP, Bernard H, Wal JM, Savelkoul HF, Wichers HJ, Mills EN. Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol Nutr Food Res. 2011; 55:1887–1894.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download