Epidemiologic studies of food allergy in children
Food allergy (FA) is a worldwide problem, with increasing prevalence in many countries, and it poses a clearly increasing health problem in Korea. There are several epidemiological studies on FA reported for the the general population, but the prevalence varies considerably due to many factors, such as use of different classification systems, biased participants, lack of confirmative diagnostic tests, rapid evolution of disease, and large numbers of potential triggers [1]. There are a few population-based studies which describe the prevalence of individual foods causing clinical symptoms, however, the majority of epidemiologic studies have inherent limitations, as a high number of FA cases are not diagnosed by a physician, which results in low grade reliance on the diagnosis of FA [2-4]. In a recent study, the overall prevalence of self-reported FA to at least 1 food was 8.07% in all ages and 7.14% in children after adjustment for several factors [2]. According to a large-scale meta-analysis in the EuroPrevall Program, based on 23 studies, the overall prevalence rates of self-reported FA were 12% in children, and 3% for all ages, on the basis of 6 studies with double-blind-placebo-controlled food challenge [5]. By a structured telephone survey in German children aged 0-17 years, 4.2% of children were FA [6] and 2.3% of 11-year-old children confirmed FA in a UK study, using clinical history plus objective assessment [7]. There are several epidemiological studies on FA in Asian countries. In the late 1990s, the reported prevalence of FA in Singaporean children aged 5-12 years was 4-5%, and the prevalence in Japanese children younger than 6 years was 12.6% [8, 9].
In Korea, as a part of the International Study of Asthma and Allergy in Childhood (ISAAC), there were a series of nation-wide population studies for prevalence of allergic disease in children, with the Korean version of ISAAC in 1995, 2000, and 2010. Using the data from surveys in 1995 and 2000, we compared the prevalence of FA in elementary and middle school aged children, between two surveys [10]. In this study, the lifetime prevalence of FA was not significantly different from 1995 to 2000 among 6-12 year-olds (10.9% in 1995 and 8.9% in 2000) or among 12-15 year-olds (11.3% in 1995 and 12.6% in 2000). The twelve-month prevalence of FA also showed no significant differences from 1995 to 2000 among both age groups (6-12 years-old, 6.5% in 1995 and 5.7% in 2000; 12-15 year-olds, 7.4% in 1995 and 8.6% in 2000). On the other hand, the mean lifetime prevalence of FA which had ever been diagnosed by a medical doctor was 4.7% among 6-12 year-olds and 5.1% among 12-15 year-olds, respectively, in 2000. As a part of the ISAAC Phase III, there was a population-based, cross sectional study in Korea, which randomly selected more than 3,000 participants among the first grade of elementary school (6-7 year-olds) and middle school children (12-13 year-olds) [11]. In this study, the prevalence of 'perceived FA, ever' was 15.0% in 6-7 year-olds and 12.5% in 12-13 year-olds, but the actual rates were 3.3% and 4.5% in each age group, respectively.
Over 90% of FA was caused by egg, cow's milk, peanut, tree nut, fish, shellfish, soy and wheat, and the prevalence of individual FA was influenced by age, culture, ethnic background and dietary pattern [12, 13]. The most frequently offending foods are egg, cow's milk, soy and peanut in children, while wheat, shellfish and tree nuts were the most commonly offending foods in adults. In a recent study by meta-analysis of 51 publications, the prevalence of self-reported FA for 5 foods (cow's milk, egg, peanut, fish and crustacean shellfish) was 12% in children [5]. Besides the common allergenic foods, several foods, such as buckwheat, mustard, sesame, kiwi fruits, are important in some countries due to the cultural dietary patterns [14-18]. In a recent population-based study of FA in Canada, cow's milk, peanut and tree nuts were the most common FA in children, and prevalence rates were 2.23%, 1.77% and 1.73%, respectively [2].
In Korean children, the major causes of FA are similar to those of other countries, though the order varies among studies. According to a large, self-reported population-based study in elementary and middle school students, foods which are well-known worldwide, including egg, cow's milk, fish, seafood, are also prevalent as causes of allergies among Korean children [10]. However, in this survey, pork, chicken, beef, and peach were also highly ranked, although the clinical relevance of these results were not fully accepted, due to the inherent limitation of the questionnaire for the self-reported study. In the study as a part of ISAAC Phase III survey in Korea, they reported the prevalence of confirmed or possible immediate-type FA, with a detailed questionnaire relating to symptoms, onset time, and detection of specific IgE antibodies relevant to the history [11]. In 6-7 year-olds with confirmed or possible immediate-type FA, the most common causative food was egg, and other common foods were crustaceans, fruits, fish, and cow's milk, in order. In contrast, crustaceans and fruits were the most frequent causative foods in 12-13 year-olds. To evaluate the prevalence of sensitization to common food allergens, we measured serum specific IgE by ImmunoCAP in young children (less than 4 years of age) with atopic dermatitis (AD) [19]. In this study, 266 AD patients aged 2-28 months were enrolled, 198 of which were moderate to severe AD patients. Using a cut-off value of over 0.35 KU/L, the rates of sensitization to egg, cow's milk, peanut and soybean were 51.5%, 31.2%, 16.2% and 15.4%, respectively. However, the positive rates which were calculated using the values of age-matched diagnostic decision indicated that the rates of sensitization for egg, cow's milk, peanut and soybean were 32.7%, 4.5%, 3.0% and 1.1%, respectively. Also, there was a small birth cohort study which determined the incidence and risk factors of immediate-type FA during first year of life in Korean infants [20]. In this study, a total of 1,177 infants, the prevalence rate of FA was 5.3% in infants, and the three leading food allergens were egg (33/62), cow's milk (20/62) and peanut/nuts (8/62). The infants with a history of maternal AD showed a significantly higher prevalence of FA.
Food-related anaphylaxis in Korean children
There is evidence that the incidence and severity of childhood FA has been increased in past 2 decades. The numbers of pediatric emergency department (ED) visits and deaths related to food induced anaphylaxis have also increased in western countries [21-23]. In children, one of the most common causes of anaphylaxis treated in emergency rooms is FA, and peanut and tree nuts are the main causes of plant food allergies resulting in fatal or near fatal forms of allergies in western countries [24-30].
In Korea, an epidemiologic study on anaphylaxis children was carried out, which was based on the data from the Korean Health Insurance Review and Assessment Service (KHIRA) from 2001 to 2007 [31]. In this study, the incidence of anaphylaxis for those under the age of 19 was 0.7-1 per 100,000 person-year. The causes of anaphylaxis based on data from KHIRA were unknown (61.7%), foods (24.9%), and drugs (12.4%) in order. Among the foods, buckwheat (29.3%) was the most common cause of anaphylaxis and the other reported foods were cow's milk (9.8%), kiwi (9.8%), pine nut (7.3%), crab (5.3%), peanut (4.9%), pupa (4.9%) and so on. In our recent epidemiologic study, we evaluated the incidence and clinical characteristics of pediatric ED visits which occurred due to severe forms of FA in Korea [32]. The data were collected for 78,889 patients of 18 years of age or younger, who visited the ED during June 2008 to Mar 2009 in 9 hospitals located in 8 cities in Korea. Based on the medical records, the frequencies, symptoms, causes, and onset times were evaluated. Out of 78,889 pediatric ED visits, food related systemic urticaria, angioedema or anaphylaxis accounted for 169 visits. Among them, 57 were anaphylaxis, where 36 of 57 (63.16%) were food related (4.56 per 10,000 pediatric ED visits). Skin symptoms (92.8%) were most common in anaphylaxis patients, and cardiovascular (29.8%), gastrointestinal (28.07%) and respiratory (24.56%) symptoms followed. Sixty percent of food related anaphylaxis cases were developed during or within 4 h of exposure. The most common causative food was fish, and cow's milk, other seafood, chicken, pork, egg, walnut, pupa, peanut, beef, buckwheat followed. However, in this study we did not identify the specific IgE antibodies related to clinical history, so the real causative effect of these foods need to be evaluated through future clinically orientated study. There was no death reported to occur from anaphylaxis in this study.
Plant FA in Korean children
Introduction of plant FA
Analysis of plant food allergen sequences showed that allergenic components belonged to only 27 of the identified food-derived more than 8,000 protein families, and these are as follows; 1) Prolamin superfamily, including prolamins, which have been identified in cereals; 2S albumins in peanut, tree nuts and mustard; non-specific lipid transfer proteins in all types of plant food and latex; and bifunctional-amylase/protease inhibitors in cereals. 2) Cupin family includes 7/8S globulins, which have been identified in legumes, nuts, seeds; and 11S globulins in peanut, tree nuts. 3) Profilin is the actin binding regular protein which has been identified in all types of plant food. 4) Bet v 1 related proteins (pathogenesis related proteins family 10, PR-10), which have also been identified in all types of plant food [33]. As mentioned above, peanut contains all of the major plant derived allergenic components, as well as sharing most of the sequences with allergenic legumes, nuts and other seeds. Since the 1990's, the use of lupine seeds has been increased, and lupine seed protein allergies have been reported and increased during 15 years. The more complex thing is that legumes (peanut, soybean, lentils, beans, chickpeas and peas) may cross-react with lupines in around 15-87% in cases, with or without clinical relevancies, so the evaluation of cross reactions, interferences or ostentations among these plant-derived foods components, is an interesting issue for future plant FA research [33]. Almost all cases of peanut allergy (PNA) is IgE-mediated disease, occurs in over 1% of children, and it has been tripled during last decade in the western countries [30, 34]. Considering the increasing incidences of severe forms of PNA, there are studies on allergen-specific immunotherapy, clinical and epidemiologic pattern of PNA, and diagnostic approaches for PNA in children without previous exposure are a rising research area, among others [29, 34-36]. In addition, fruits, grains and other plant derived food allergens are more important in some regions than in others. For example, sesame has been highlighted as a potent plant food allergen, and buckwheat flour is well known as a potent food allergen in Japan, Korea and several European countries, and the kiwi fruits are the important allergenic foods in some countries [14, 15, 17, 37, 38].
Peanut and walnut allergy in Korean children
Although PNA is not prevalent in Asia as it is in the western countries, during recent 15 years, the incidences of PNA has been increased in Korea, especially in children. We evaluated the clinical symptoms and history of PNA and serum specific IgE to peanut and other major food allergens (ImmunoCAP; Phadia, Sweden) in 804 patients under the age of three [39]. The patients had moderate-to-severe atopic dermatitis, and were enrolled from January 2000 to December 2006 at Ajou University Hospital. Among them, 148 showed sensitization to peanut (>0.35 kU/L), and were then analyzed in detail based on information recorded on their patient logs, as well as additional information gathered through telephone surveys. For the clinical estimation of PNA, we used the cut-off values of peanut specific IgEs ≥ 15 kU/L. Our study reveals that the peanut sensitization rate in Korea reaches 18%, and that patients showed significant tendency to have co-sensitization with house dust mites, egg white, wheat, and soybean, when sensitized to peanut. The higher specific IgE to peanut was related to the likelihood of the patient to develop severe systemic reactions.
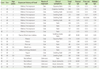
Walnut allergy (WNA) is also an important plant FA and is reported mainly in the western countries, while it is rarely reported in Asia. However, recently we reported a relatively large clinical study on WNA in Korean Children [40]. In the study, we focused on the clinical characteristics of WNA and co-senstization/cross-reactivity between walnut, peanut and pine nut in young Korean children (Table 1). This study is based on the analysis of data from 69 patients under 4 years of age, who have been suspected of having WNA and diagnosed with allergic disease at Ajou University Hospital, Suwon, from January 2009 to December 2010. Sera of all patients were analyzed for serum peanut-specific IgE, walnut-specific IgE, and pine nut-specific IgE (ImmunoCAP; Phadia, Sweden). Clinical details of patients were collected by medical history. In addition, we had produced walnut, peanut, and pine nut extracts, and ELISA inhibition tests were applied to the sera of patients. Twenty-nine (42%) were accompanied with atopic dermatitis, 15 (21.7%) with urticaria, 10 (14.5%) with anaphylaxis, 6 (8.7%) with angioedema, and 1 (1.5%) with asthma. Twenty-two (31.9%) were sensitized to WN (>0.35 kU/L, 0.45-27.4 kU/L), and among them, 10 (45.5%) were sensitized only to WN, 6 (27.3%) were co-sensitized with PN, 6 (27.3%) were co-sensitized with PN and pine nut. Using the IgE ELISA inhibition test, we confirmed that PN and WN has partial cross-reactivity with IgE binding capacity. Further investigation has been carried out for 31 selected clinical WNA cases aged 8~108 months, and sera from 26 patients were used for component-resolved diagnosis (CRD) by protein microarrays (ImmunoCAP ISAC-CRD 103; Phadia, Sweden). Jug r 1 is the only major allergenic component in Korean children with clinical WNA, and 40% of patients were co-sensitized to various nuts, without typical clinical manifestations [41].
Studies on buckwheat and rice allergy in Korean children
There have been several studies on allergies to grains, such as buckwheat, rice and barley, in Korean children [42-46]. Buckwheat (BW) is considered to be one of the most important food allergens, and positive skin tests are found in about 5% of Korean children [17]. We investigated the positive and negative predictive values of BW-specific IgE in 28 children with challenge proven BW allergy and 16 asymptomatic control subjects, with positive skin test to BW [42]. According to the receiver operator characteristic analysis, the optimal cut-off level of BW-specific IgE, as the definitions of serum BW-specific IgE positive, was 1.26 kUA/L. With this selected cut off level, the sensitivity, specificity, positive and negative predictive values were 93.10, 93.33, 79.75 and 97.96%, respectively. Furthermore, we reported that 3 cases of nocturnal asthma in Korean children who used BW chaff-stuffed pillow for 3-7 years [43]. In all patients, BW sensitization was initiated by the BW chaff-stuffed pillow, and all three children showed positive skin reactions to BW, but none of them had positive reactions to house-dust mites. Nocturnal asthmatic symptoms were completely recovered during 7 days of pillow elimination, and asthmatic responses were provoked by bronchial challenge with BW extract. Thus in this study, we confirmed that a small amount of BW attached to chaff-stuffed pillow can induced BW sensitization, provoke asthma symptoms in children. And there was an immunological study on the identification and characterization of major allergenic components in Korean children and adults with BW allergy [44]. In this study, 19 BW allergic subjects with symptoms after BW ingestion and 15 asymptomatic control subjects with positive skin prick test to BW were recruited. BW-specific IgE was measured with the Pharmacia CAP system and allergenic components of BW were analyzed using two-dimensional PAGE, and sequencing of N-terminal amino acids. From the BW-allergic patients, the sensitivity (100%), specificity (53%), and negative (100%) and positive predictive values (73%) of Pharmacia CAP specific IgE for diagnosis were estimated. And the major allergenic components which bind to more than 50% of patients' sera were 24-kDa (pI 8.3), 16-kDa (pI 5.6), and 9-kDa (pI 5.0/6.0). However, the specific IgE to split 19-kDa (pI 6.5/7.0) allergens were more specifically found in BW-allergic patients than in the asymptomatic subjects. N-terminal amino-acid sequences of 19-kDa and 16-kDa allergens showed moderate and weak homology to the 19-kDa globulin protein of rice and amylase/trypsin inhibitor of millet, respectively. The N-terminus of the 9-kDa isoallergens were not different from each other and were identified as the reported trypsin inhibitors of BW. Attenuation of the IgE binding to the 9-kDa allergen was found with periodate oxidation.
Rice is a basic food which rarely causes FA in Korea. However there has been an increase of non-specific sensitization in Korean children, without clinical symptoms. So, we tried to determine the major IgE binding components in rice extract and their clinical significance [45]. Twenty-four children (15 boys and 9 girls; mean age, 16.3 months) with allergic disease, who were sensitized to rice antigen confirmed by ImmunoCAP in the Pediatric Allergy Respiratory Center at Soonchunhyang University Hospital, were enrolled. In this study, the IgE binding capacities of various types of rice (raw, cooked, and heat-treated, simulated gastric fluid, and simulated intestinal fluid) were investigated using SDS-PAGE and IgE immunoblot analysis. In SDS-PAGE, more than 16 protein bands were observed in the raw rice, whereas only 14-16 kDa and 31-35 kDa protein bands were observed in cooked rice. The common SDS-PAGE protein bands observed in SGF-, SIF-, and heat treated rice were 9, 14, and 31 kDa. In a heated-rice IgE immunoblot, protein bands of 9, 14, and 31-33 kDa were found in 27.8%, 38.9%, and 38.9% of all sera, respectively. Furthermore, the 9 kDa, 14 kDa and 31-kDa bands were IgE binding components in 50%-75% of sera from symptomatic rice allergic children, and hence, appeared to be the clinically relevant rice allergens in Korean children.
Other plant food allergies in Korean children
Recently we experienced 3 cases of barley allergic infants who became sensitized due to their infant diet containing barley flour. Among them, we reported the case of an 11-month old boy who experienced an episode of anaphylaxis due to ingestion of barley flour. [46]. The infant experienced generalized urticaria, periorbital swelling, wheezing and dyspnea 30 min after eating baby food powder, containing barley, chestnut, and rice. Laboratory tests demonstrated high total serum IgE (531 kU/L) and revealed high levels of IgE sensitizations to wheat (64.3 kU/L), barley (35.2 kU/L), and rice (1.39 kU/L), but the patient only had a clinical reaction to barley. Using the IgE-immunoblot assay, 7, 14, 25, and 26 kDa of IgE binding bands were identified, and a high degree of IgE crossreactivity between barley and wheat extracts were revealed using IgE-immunoblot inhibition and ELISA-inhibition tests. We recently evaluated the clinical characteristics of kiwi fruit allergy as well as kiwi specific IgE in Korean young children [47]. The study was based on data analysis of 18 patients, aged 11-108 months (median age 25 months), who were diagnosed with clinical kiwi fruit allergy at Ajou University Hospital from June 2005 to June 2012. Clinical details were collected by medical history and telephone survey. Twelve out of 18 (66.7%) were diagnosed with angioedema or urticaria, 4 (22.2%) were diagnosed with oral allergy syndrome, 1 was presented with dyspnea, and 1 was diagnosed with anaphylaxis by kiwi fruit. Oral route of exposure (88.9%) was most common and 89% of subjects experienced systemic reactions at the time of first exposure. Hence, from this study, we have established that kiwi fruit allergy is not rare in Korean children, and that systemic reactions are more common in younger patients. The kiwi fruit allergy in older children who are allergic to pollens and kiwi together should be evaluated.
In summary, although the systematic studies have not been performed as of yet, plant-derived food allergies are not rare in Korean children and clinical symptoms are comparably severe. Even in infants and young children, the allergies due to plant foods (such as soy, peanut, walnut and other tree nuts, wheat flour, buckwheat flour and other cereals, kiwi fruit and other fruits) are not rare, and the face that their prevalence seems to be increasing demands further consideration.
Allergic food labeling system in Korea and consumers' awareness
With the safety concerns related to common food allergens, "food labeling systems" have been operated in many countries, including the Canada, US, UK, Japan and Korea. It is also an important issues for education as well as regulation among the public. Allergic food labeling systems for 12 foods (eggs, cow's milk, buckwheat flour, peanut, soybean, wheat flour, mackerel, crab, shrimp, pork, peach, tomato) have been operated in Korea since 2004. To evaluate consumer's awareness of this food labeling system, we developed a questionnaire for analysis of public awareness. Through evaluating the data from a total of 993 subjects from 7 university hospitals, including 337 of food allergic subjects (FAS) and 656 of non-allergic subject (NAS), we evaluated the awareness of this labeling system and consumers' priority of needs for labeling, using 9 items of questions [48]. As a result, we defined 38.3% of FAS and 18.6% of NAS read labels and 79.2% of FAS and 70.1% of NAS had problems with use of labels due to difficulties, complexities and several other inconveniences. The major causes of FA were egg, peach, milk, shrimp, pork, crab, mackerel, peanut and others. Among the 12 foods enrolled in the labeling system in Korea, consumers' choice of the top 5 priority foods requiring labeling were egg, milk, peanut, mackerel and pork in food allergic subjects, while egg, milk, mackerel, pork, peanut were the highest ranked in non-food allergic subjects. We confirmed that there is low consumers' awareness to the allergenic food labeling system, and that the accessibility of this system should be improved.
CONCLUSION
FA is a worldwide problem with increasing prevalence in many countries, and it poses a clearly increasing health problem in Korea. The incidence of FA in Korean children has been reported by several large scaled epidemiologic surveys, although, there are several general limitations of such studies, mainly caused by inherent flaws within self-reported surveys. We need further studies of infant and preschool aged young children. Recently, a study group for atopic dermatitis and FA, a subcommittee in the Korean Academy of Pediatric Allergy and Respiratory Disease, has initiated a nation wide survey of anaphylaxis, and we expect that useful data and information will be elicited from this survey. Furthermore, detailed systematic studies on plant food allergies which cause severe reactions in Korean children have been undertaken in clinical and immunological research fields, and they can provide a valuable guide to Korean children with FA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download