Abstract
Allergic rhinitis (AR) is the most common chronic pediatric disorder. The International Study for Asthma and Allergies in Childhood phase III found that the global average of current rhinoconjunctivitis symptoms in the 13-14 year age-group was 14.6% and the average prevalence of rhinoconjunctivitis symptoms in the 6-7 year age-group was 8.5%. In addition to classical symptoms, AR is associated with a multidimensional impact on the health related quality of life in children. AR affects the quality of sleep in children and frequently leads to day-time fatigue as well as sleepiness. It is also thought to be a risk factor for sleep disordered breathing. AR results in increased school absenteeism and distraction during class hours. These children are often embarrassed in school and have decreased social interaction which significantly hampers the process of learning and school performance. All these aspects upset the family too. Multiple co-morbidities like sinusitis, asthma, conjunctivitis, eczema, eustachian tube dysfunction and otitis media are generally associated with AR. These mostly remain undiagnosed and untreated adding to the morbidity. To compound the problems, medications have bothersome side effects which cause the children to resist therapy. Children customarily do not complain while parents and health care professionals, more often than not, fail to accord the attention that this not so trivial disease deserves. AR, especially in developing countries, continues to remain a neglected disorder.
The Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 updated document estimates that there are 500 million subjects in this world who suffer with allergic rhinitis (AR) [1]. Data suggests that AR is the most common chronic disorder in the pediatric population with up to 40% of children affected [1]. The disease along with associated co-morbidities has a profound impact on the daily lives of children. Irritability, sadness, impairment of sleep and limitation of activities at school as well as home are often seen in these children. AR results in day-time fatigue and impairment of cognition and memory in children which significantly affect the learning process and thus impacts on school performance and all these aspects upset the family [2]. Many of these problems go completely unnoticed as children often fail to share them at home or at school. Furthermore, adverse effects of medications used for treatment of AR often compound these problems [2]. Although AR greatly impacts life at home, school and even while sleeping, it is treated as a trivial and a commonplace disorder. Consequently, it does not receive the attention it deserves from the patient, the family as well as the health care professionals, especially in developing countries like India [3].
The International Study for Asthma and Allergies in Childhood (ISAAC) phase III [4] found that the global average of current rhinoconjunctivitis symptoms in the 13-14 year age-group was 14.6% and the average prevalence of rhinoconjunctivitis symptoms in the 6-7 year age-group was 8.5%. The prevalence of AR in certain centres approached more than 50%. Higher prevalence of severe rhinoconjunctivitis was seen in lower and middle income countries particularly in Africa and Latin America [4]. In India, the ISAAC phase I [5] revealed that 12.5% children in the 6-7 year age-group and 18.6% in the 13-14 year age-group had nasal symptoms alone, while allergic rhinoconjunctivitis was seen in 3.2% and 6.3% children in these age-groups respectively. In ISAAC phase III [4], the prevalence of current nose symptoms increased to 12.9% and 23.6% in the 6-7 and 13-14 year age-groups respectively, while the prevalence of allergic rhinoconjunctivitis increased to 3.9% and 10.4% respectively. Among the Asia-Pacific countries, the ISAAC phase III data [4] revealed that allergic rhinoconjunctivitis was lowest in Indonesia with prevalence ranging from 3.6% in the 6-7 year age-group to 4.8% in the 13-14 year age-group. In contrast, the highest prevalence was documented in Taiwan which ranged from 24.2% in the 6-7 year age-group to 17.8% in the 13-14 year age-group [4]. Most countries in this region showed an increase in the prevalence of allergic rhinoconjunctivitis between the ISAAC phase I and III studies (Table 1) [6]. In an 11 Asian countries study in selected centers, the prevalence of AR ranged from 10 to 46% in children and was more common in boys [7]. Several studies of AR in children from Asia confirm the enormity of burden of this disease. In Taiwan, the mean one-year and overall 8-year (2000 to 2007) prevalence of AR in children and adolescents was 11.3% and 37.8% respectively [8]. Similarly in China, a study in 24,290 children showed that the prevalence ranged from 7.83% to 20.42% [9]. However, the ISAAC phase III study in Tibet [10] revealed that allergic rhinoconjunctivitis was present in 5.2% of the 3196 children in the 13-14 year age-group. The authors state that this was the lowest prevalence in the ISAAC phase III study worldwide [10].
The ARIA update 2008 [1] classifies AR on the basis of frequency and severity. Mygind [11] first proposed classifying AR on the basis of predominant clinical symptoms. He proposed calling those with predominant blockage as 'blockers' and those with runny nose as 'sneezers and runners' [11]. We sketched the profile of these two clinical presentations in 114 adults with AR [12] and found that almost two-third 72/114 (63%) were 'sneezers and runners' while 42/114 (37%) were 'blockers'. 'Sneezers and runners' had significantly more sneezing, rhinorrhoea, itchy nose, eyes and palate. This group had a significantly more family history of atopy, seasonal disease and sensitivity to seasonal allergens like pollen. In contrast, 'blockers' had significantly more nasal blockade, thick nasal mucus, and post nasal drip. In addition, 'blockers' had significantly more sensitisation to perennial allergens like fungi and house dust mite and had perennial disease [12]. Recently, we evaluated 126 school going children with AR and/or asthma, of whom 14 (11.1%) had AR only, 100 (79.3%) had concomitant AR and asthma, while 12 (9.5%) had only asthma. On categorisation, 46 (40.4%) were classified as 'sneezers and runners' while 68 (59.6%) were classified as 'blockers'. 'Sneezers and runners' had more sneezing (100% vs. 85.3%) and itchy nose (63% vs. 54.4%), while 'blockers' had more persistent disease (52.9% vs. 32.6%), post nasal drip (67.6% vs. 54.3%), loss of smell (22.1% vs. 10.9%), loss of taste (20.6% vs. 10.9%) and nasal quality of voice (14.7% vs. 4.3%). However, the differences did not achieve significance. On CT-PNS, sinusitis (Fig. 1) was recorded in 78/126 (61.9%) children [13].
Both adults and children with AR have disturbances in sleep. Although the exact mechanisms of sleep impairment due to AR is not known, uncontrolled symptoms especially nasal congestion are thought to be responsible. The medications used may also compound the problems [14-17]. In a study in 39 children with habitual snoring, 14 (36%) had a positive radioallergosorbent test for allergens [14]. Children with atopy had higher prevalence of obstructive sleep apnea syndrome (OSAS) (57% vs. 40%; x2 = 9.11, p < 0.01). The authors postulated that allergy was a risk factor for the presence of OSAS in the children [14]. A questionnaire based survey in Greece [15] involving parents of 3,680 children revealed that habitual snoring was present in 5.3%, 4%, and 3.8% in the children of 1-6, 7-12, and 13-18 year age-groups respectively. This study also found that chronic rhinitis was one of the most important risk factors for habitual snoring (odds ratio [OR] = 2.1, confidence interval [CI] = 1.6-2.7). Intranasal corticosteroids (INCS) usage in children with AR appears to have an objective improvement in sleep parameters on polysomnography with a decrease of mean number of sleep arousals per h from a baseline of 8.4 to 1.2 (p = 0.005) [16]. In our study [13], parents reported sleep disturbances in 70/114 (61.4%) school going children with AR. Problems with sleep would heighten daytime somnolence and impair cognition which in turn may result in behavioral problems [2, 17, 18].
The symptoms of AR like nasal blockade, itching, rhinorrhoea and sneezing cause severe distraction during class hours. Uncontrolled symptoms at night leading to sleep loss and secondary daytime fatigue may also contribute to learning impairment similarly. Apart from absenteeism from the class, even when present during class hours, the child has decreased productivity. Complications of AR like sinusitis, eustachian dysfunction and associated conductive hearing loss may enhance the learning dysfunction [2]. Irritability, distraction, fatigue increase absenteeism and along with embarrassment at the school result in impaired school performance in these children. This can be compounded by the side effects of medication used for AR. The recreational activities of children with AR are often limited which leads to diminished social interaction and consequent isolation [2, 17]. Even in adults with AR there is slow speed of cognitive processing and impairment in working memory during the ragweed pollen season [18].
The presence of sleep abnormalities in AR along with nocturnal snoring and hypoxia also affect the school performance of a child [2, 19]. Allergy and daytime nasal obstruction are independent risk factors for sleep disordered breathing ranging from habitual snoring to obstructive sleep apnoea [14, 15]. A study [19] on 1,144 school children assessed the association of habitual snoring, intermittent hypoxia and academic performance. Of 1,129 children in whom information on snoring was available, 410 (36.3%) never snored, 605 (53.6%) snored occasionally, 89 (7.9%) snored frequently and 25 (2.2%) children always snored. The "always snorers" had poorer academic performance in mathematics (OR: 3.6, 95% CI: 1.3-10.1), science (OR: 4.3, 95% CI: 1.3-14.6), and spelling (OR: 3.5, 95% CI:1.2-10.3). "Frequent snorers" had poorer academic performance in mathematics (OR: 2.4, 95% CI: 1.3-4.7) and spelling (OR: 2.0, 95% CI: 1.04-3.8). This study showed a significant association between snoring, with or without hypoxia, and poor academic performance [19]. It is therefore not surprising, that a study done on 9,538 adolescent students in 2007 revealed that patients with nasal symptoms severe enough to cause activity limitation had poorer grades especially those on anti-allergy medications as compared to normal subjects [20].
We used the Work Productivity and Activity Impairment Questionnaire - Allergy Specific to study the activity impairment at school and home in 114 school-going children with AR. The mean percent of class hours missed by children in the preceding week as a result of the symptoms of AR was 11.6 h (SD ± 20.0). Even when present in class, children with AR suffered a mean impairment of 36.8% (SD ± 20.6). In total, the mean classroom impairment was 41.52% (SD ± 23.7). At home the children suffered a 35% (SD ± 20.7) work impairment. This highlights the enormity of activity limitation in children as reflected by the class time missed and impairment during class hours as a result of AR [13]. This not so trivial disease is responsible for a huge loss of work productivity and absenteeism from school. In 1994, in USA alone, AR accounted for approximately 824,000 missed school days in children [21].
There is an immense difference between the level of actual disability that the child faces and that what is perceived by the care-givers and treating physicians. Allergic rhinitis too encompasses a spectrum of problems that go beyond the classical symptoms of the disease, and are best assessed by measuring the health related quality of life (QOL). Various disease specific questionnaires [22, 23] have been developed for the different aspects of QOL issues in children and adolescents with AR viz. physical, emotional, sleep problems and activity limitation. Assessment of these parameters help in knowing patients' own perception of the disease, and help provide vital information which cannot be obtained from conventional clinical and functional tools. Though the impact of AR on QOL is multidimensional, the impairment is more in the physical domains than in the psychosocial domains of health [24-26]. Young children were less disturbed psychologically and emotionally, but were more troubled by taking medications and carrying tissues to blow their noses [24-26]. During a cross sectional analysis [26], the responses of parents or caregivers as well as the responses of 23 children and adolescents with AR were compared to those of normal age-matched subjects using the generic Child Health Questionnaire (CHQ-PF50). The patients' scores were found to be lower than that of the normal subjects in both the physical and the psychosocial summaries of the CHQ-PF50. This difference was more in the physical than in the psychosocial summary score. The investigators concluded that AR had a global negative impact on the QOL with a profound effect on physical aspect of the health in such patients. In addition, a negative effect on family relations was also observed [26].
The impairment in QOL in patients with AR increases during the pollen season in both seasonal as well as perennial AR. In seasonal AR, QOL is worse during the pollen season, as compared to perennial rhinitis [27]. Another study [28] involving 84 children, in the age-group of 6 to 17 years, with seasonal allergic rhinoconjunctivitis, asthma and/or cutaneous manifestations were assessed before and during the grass pollen season using the pediatric allergic disease quality of life questionnaire. The health related QOL significantly correlated with the average pollen count in the previous week (regression coefficient 0.038, 95% CI 0.027-0.049, p < 0.001) [28].
The productivity and ability to perform day-to-day activities in both children and adults is severely affected with a profound impact on the emotional aspects of the life [2, 17, 24-28]. Children with AR are unable to integrate socially and experience a feeling of isolation even within their families. This may be due to decreased participation in family events due to AR, often resulting in family dysfunction [2, 17].
Usually a part of a wide spectrum of systemic allergic diseases, AR rarely occurs in isolation and is associated with multiple co-morbidities. A close relation between AR and asthma is well documented [29]. Symptoms of rhinitis were observed in 28 to 78% of asthmatics, while 17-38% patients with AR had concomitant asthma [30]. A questionnaire based study conducted by us [31] showed that 75% of 405 children with asthma had coexistent rhinitis. Simultaneous occurrence of both the diseases was recorded in about three fourths of these children [31]. A retrospective analysis [32] has shown that children with AR younger than 7 years were at a 2 to 7 times greater risk of developing asthma. The occurrence of AR in children also increased the risk of persistence of childhood asthma by middle age [32].
In the ISAAC phase III study in the Indian subcontinent [4], among the 50,106 children in the age-group of 6-7 years, 1,183 (2.4%) had symptoms of rhinoconjunctivitis alone, 554 (1.1%) had symptoms of both rhinoconjunctivitis and asthma, 174 (0.3%) had symptoms of rhinoconjunctivitis and eczema, and 174 (0.3%) had symptoms of all the three conditions. Among the 55,815 children in the age-group of 13-14 years, 4,177 (7.5%) children had symptoms of rhinoconjunctivitis alone, 900 (1.6%) had symptoms of both rhinocinjunctivitis and asthma, 496 (0.9%) had symptoms of rhinoconjunctivitis and eczema, and 395 (0.70%) had symptoms of all three conditions. In other countries of the Asia Pacific region, of the 60,052 children in the age-group of 6-7 years, 3,520 (6.2%) children had symptoms of rhinoconjunctivitis alone, 1,117 (2.0%) had symptoms of both rhinocinjunctivitis and asthma, 884 (1.5%) had symptoms of rhinoconjunctivitis and eczema, and 494 (0.9%) had symptoms of all three conditions. Similarly in the age-group of 13-14 years, among the 99,688 children, 8,581 (9.3%) children had symptoms of rhinoconjunctivitis alone, 2,135 (2.3%) had symptoms of both rhinocinjunctivitis and asthma, 1,064 (1.1%) had symptoms of rhinoconjunctivitis and eczema, and 530 (0.60%) had symptoms of all three conditions [4].
As highlighted in the ISAAC study, AR is commonly associated with conjunctivitis [4, 6]. The exact prevalence of allergic conjunctivitis in children with AR cannot be determined as the patients usually do not self report eye symptoms and do not attach much importance to it [1]. A study from China revealed that 430/485 (89%) children with AR had concomitant allergic conjunctivitis [33]. Allergen exposure of nasal or conjunctival mucosa may lead to inflammation at both the places probably due to anatomical contiguity. Intranasal corticosteroids have been shown to suppress nasal as well as ocular symptoms [34].
Patients with perennial AR are at a larger risk of developing sinusitis [35]. At our Institute, we found that 136/189 (72%) of subjects with AR had concomitant sinusitis [36]. The presence of sinusitis increased the morbidity in patients with AR especially in 'blockers' and increased the incidence of postnasal drip (62/88 vs. 15/43, p < 0.05) as well as sneezing (52/88 vs. 7/43, p < 0.05) [36]. Since sinusitis rarely occurs without rhinitis, the term 'rhinosinusitis' is frequently being used interchangeably with the term 'rhinitis' [1]. Patients with AR also have a higher incidence of nasal polyposis which is considered to be a part of spectrum of chronic sinus pathology [37].
Allergy should be investigated in children with symptomatic adenoid hypertrophy [1]. Although the exact role of allergy in adenoid hypertrophy is unknown; the presence of sensitisation to inhalant allergens has been reported to alter the immunology of adenoid tissue which might have an aetiological role in adenoid hypertrophy [38]. Inflammation in AR can lead to mucosal swelling around eustachian tube. Tympanometery performed in 80 patients with AR and 50 healthy controls, comprising adults and children, demonstrated abnormalities in 15.5% of children below 11 years of age with AR [39]. In contrast, no abnormal curves were seen in healthy controls; thereby demonstrating that children with AR are at a greater risk of Eustachian dysfunction [39]. The presence of rhinitis or atopic eczema is significantly associated with a higher incidence of otitis media with effusion [38, 40].
The goals of management of AR, as described in the ARIA management pocket reference guide [41], include (i) no troublesome symptoms, (ii) performance of near normal daily activities without school absenteeism, (iii) no sleep impairment, and (iv) minimal or no side-effects of treatment. Allergen avoidance along with pharmacotherapy is the mainstay of treatment. Key allergens should be identified and avoided as far as possible. Oral/intranasal antihistamines along with INCS comprise the armamentarium against AR [1, 41]. However, self medication is very common, leading to both over as well as under-medication. While over-medication is associated with unnecessary costs and numerous side effects leading to increased morbidity, under-medication leads to suboptimal control of symptoms which hampers quality of life [1, 2, 20, 21].
Currently, oral antihistamines are usually the first group of drugs to be prescribed in the pediatric population [1]. Since the first generation antihistamines cause sedation, drowsiness and anti-cholinergic side effects [42], they are best avoided lest they lead to impairment in performance and learning at school. The newer second generation antihistamines, which include cetirizine/levocetirizine, loratadine/desloratadine and fexofenadine, have now emerged as the preferred drugs [1, 42].
Intranasal corticosteroids are the most effective form of therapy available till date [1]. The common INCS available are beclomethasone, triamcinolone, budesonide, fluticasone, mometasone and ciclesonide [1]. INCS have shown to improve nasal congestion and have demonstrated a reduction in sleep problems and daytime sleepiness among patients [43]. This is bound to improve quality of life during the day, reduce fatigue and eventually improve school performance in children [2, 43]. However, dry nose, mucosal crusting and bleeding are not uncommon [44]. Other adverse effects of INCS include transient symptoms of nasal stinging, throat irritation, and even nasal septal perforation [45]. Due to undue fear of systemic effects and growth reduction, parents tend to avoid INCS for their wards. Growth suppression in children due to INCS remains debatable though there is some evidence of its association with beclamethasone [46].
AR, the commonest chronic pediatric disorder, is associated with a number of comorbidities and complications and is strongly linked with asthma. AR in children has a significant impact on the quality of life, negatively affects the family and impairs the process of learning. Irrational treatment in the form of under-treatment, over-treatment as well as use of inappropriate drugs, compound the problem. This is especially true for school going children in whom timely and appropriate treatment could possibly avoid the immense morbidity encountered with this disease.
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160. PMID: 18331513.
2. Jáuregui I, Mullol J, Dávila I, Ferrer M, Bartra J, del Cuvillo A, Montoro J, Sastre J, Valero A. Allergic rhinitis and school performance. J Investig Allergol Clin Immunol. 2009; 19(Suppl 1):32–39.
3. Shah A, Pawankar R. Allergic rhinitis and co-morbid asthma: perspective from India -- ARIA Asia-Pacific Workshop report. Asian Pac J Allergy Immunol. 2009; 27:71–77. PMID: 19548632.
4. Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009; 64:123–148. PMID: 19132975.

5. Beasley R, Keil U, von Mutius E, Pearce N. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998; 351:1225–1232. PMID: 9643741.

6. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743. PMID: 16935684.

7. Pawankar R, Bunnag C, Gendeh B. Allergic rhinitis: incidence, treatment strategies, practice guidelines and co-morbidities in Asia. Presented at the 6th Asian Research Symposium in Rhinology. 2001. Hong Kong.
8. Hwang CY, Chen YJ, Lin MW, Chen TJ, Chu SY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: a national study 2000 to 2007. Acta Derm Venereol. 2010; 90:589–594. PMID: 21057741.
9. Zhao J, Bai J, Shen K, Xiang L, Huang S, Chen A, Huang Y, Wang J, Ye R. Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health. 2010; 10:551. PMID: 20836838.

10. Droma Y, Kunii O, Yangzom Y, Shan M, Pingzo L, Song P. Prevalence and severity of asthma and allergies in schoolchildren in Lhasa, Tibet. Clin Exp Allergy. 2007; 37:1326–1333. PMID: 17845413.

11. Mygind N. Mygind N, editor. Perennial rhinitis. Nasal allergy. 1979. 2nd ed. Oxford: Blackwell Scientific Publications;p. 224–232.
12. Khanna P, Shah A. Categorization of patients with allergic rhinitis: a comparative profile of "sneezers and runners" and "blockers". Ann Allergy Asthma Immunol. 2005; 94:60–64. PMID: 15702818.

13. Mir E. Assessment of health-related quality of life and work productivity in school going children with allergic rhinitis and/or asthma. 2011. Delhi: Vallabhbhai Patel Chest Institute;Doctoral dissertation submitted to the University of Delhi.
14. McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997; 111:170–173. PMID: 8996012.

15. Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, Agorogiannis E, Christodoulou S, Pantazidou A, Gourgoulianis K, Molyvdas PA. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol. 2004; 37:499–509. PMID: 15114550.

16. Mansfield LE, Diaz G, Posey CR, Flores-Neder J. Sleep disordered breathing and daytime quality of life in children with allergic rhinitis during treatment with intranasal budesonide. Ann Allergy Asthma Immunol. 2004; 92:240–244. PMID: 14989393.

17. Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001; 108:S45–S53. PMID: 11449206.

18. Marshall PS, O'Hara C, Steinberg P. Effects of seasonal allergic rhinitis on selected cognitive abilities. Ann Allergy Asthma Immunol. 2000; 84:403–410. PMID: 10795648.

19. Urschitz MS, Guenther A, Eggebrecht E, Wolff J, Urschitz-Duprat PM, Schlaud M, Poets CF. Snoring, intermittent hypoxia and academic performance in primary school children. Am J Respir Crit Care Med. 2003; 168:464–468. PMID: 12773324.

20. Sundberg R, Torén K, Höglund D, Aberg N, Brisman J. Nasal symptoms are associated with school performance in adolescents. J Adolesc Health. 2007; 40:581–583. PMID: 17531771.

21. Malone DC, Lawson KA, Smith DH, Arrighi HM, Battista C. A cost of illness study of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997; 99:22–27. PMID: 9003207.

22. Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994; 93:413–423. PMID: 8120268.

23. Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998; 101:163–170. PMID: 9500748.

24. Bousquet J, Bullinger M, Fayol C, Marquis P, Valentin B, Burtin B. Assessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 Health Status Questionnaire. J Allergy Clin Immunol. 1994; 94:182–188. PMID: 8064070.

25. Shedden A. Impact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online survey. Treat Respir Med. 2005; 4:439–446. PMID: 16336028.
26. Silva CH, Silva TE, Morales NM, Fernandes KP, Pinto RM. Quality of life in children and adolescents with allergic rhinitis. Braz J Otorhinolaryngol. 2009; 75:642–649. PMID: 19893929.

27. Maksimović N, Janković S, Tomić-Spirić V, Marinković J. Health-related quality of life assessment in patients with allergic rhinitis. Srp Arh Celok Lek. 2005; 133:223–228. PMID: 16392276.

28. Roberts G, Mylonopoulou M, Hurley C, Lack G. Impairment in quality of life is directly related to the level of allergen exposure and allergic airway inflammation. Clin Exp Allergy. 2005; 35:1295–1300. PMID: 16238788.

29. Shah A. Rarely does one hear a wheeze without a sneeze. Indian J Chest Dis Allied Sci. 2000; 42:143–145. PMID: 11089317.
30. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008; 122:S1–S84. PMID: 18662584.

31. Shah A, Panjabi C, Maurya V. Asthma and rhinitis: the co-occurrence in Delhi. Proceedings of the Third Malaysian Congress of Allergy and Immunology. 2002. Kuala Lumpur: Malaysia. p. 54. [Abstract].
32. Burgess JA, Walters EH, Byrnes GB, Matheson MC, Jenkins MA, Wharton CL, Johns DP, Abramson MJ, Hopper JL, Dharmage SC. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007; 120:863–869. PMID: 17825896.
33. Qiao T, Hu Y, Wang Z. Pediatric allergic conjunctivitis and allergic rhinitis. J Nanjing Med Univ. 2008; 22:183–187.

34. Bielory L. Allergic conjunctivitis and the impact of allergic rhinitis. Curr Allergy Asthma Rep. 2010; 10:122–134. PMID: 20425504.

35. Berrettini S, Carabelli A, Sellari-Franceschini S, Bruschini L, Abruzzese A, Quartieri F, Sconosciuto F. Perennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factors. Allergy. 1999; 54:242–248. PMID: 10321560.

36. Sahay S, Bhargava SK, Shah A. Co-occurrence of sinusitis in patients with asthma and/or allergic rhinitis in Delhi, India. Proceedings of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI) and World Allergy Organisation (WAO) Joint Congress 2006 and the 9th West Pacific Allergy Organisation. 2006. Seoul: Korea. p. 263. [Abstract].
37. Fokkens W, Lund V, Bachert C, Clement P, Helllings P, Holmstrom M, Jones N, Kalogjera L, Kennedy D, Kowalski M, Malmberg H, Mullol J, Passali D, Stammberger H, Stierna P. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy. 2005; 60:583–601. PMID: 15813802.

38. Nguyen LH, Manoukian JJ, Sobol SE, Tewfik TL, Mazer BD, Schloss MD, Taha R, Hamid QA. Similar allergic inflammation in the middle ear and the upper airway: evidence linking otitis media with effusion to the united airways concept. J Allergy Clin Immunol. 2004; 114:1110–1115. PMID: 15536418.

39. Lazo-Sáenz JG, Galván-Aguilera AA, Martínez-Ordaz VA, Velasco-Rodríguez VM, Nieves-Rentería A, Rincón-Castañeda C. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005; 132:626–629. PMID: 15806058.

40. Caffarelli C, Savini E, Giordano S, Gianlupi G, Cavagni G. Atopy in children with otitis media with effusion. Clin Exp Allergy. 1998; 28:591–596. PMID: 9645596.

41. Bousquet J, Reid J, van Weel C, Baena Cagnani C, Canonica GW, Demoly P, Denburg J, Fokkens WJ, Grouse L, Mullol K, Ohta K, Schermer T, Valovirta E, Zhong N, Zuberbier T. Allergic rhinitis management pocket reference 2008. Allergy. 2008; 63:990–996. PMID: 18691301.

42. Ten Eick AP, Blumer JL, Reed MD. Safety of antihistamines in children. Drug Saf. 2001; 24:119–147. PMID: 11235817.

43. Craig TJ, Hanks CD, Fisher LH. How do topical nasal corticosteroids improve sleep and daytime somnolence in allergic rhinitis? J Allergy Clin Immunol. 2005; 116:1264–1266. PMID: 16337455.

44. Passalacqua G, Albano M, Canonica GW, Bachert C, Van Cauwenberge P, Davies RJ, Durham SR, Kontou-Fili K, Horak F, Malling HJ. Inhaled and nasal corticosteroids: safety aspects. Allergy. 2000; 55:16–33. PMID: 10696853.

45. Deepak D, Panjabi C, Gudwani S, Chaudhary N, Shah A. Nasal septal perforation in a patient with allergic bronchopulmonary aspergillosis and rhinitis on long term corticosteroids. Asian Pac J Allergy Immunol. 2001; 19:287–290. PMID: 12009079.
46. Skoner DP, Rachelefsky GS, Meltzer EO, Chervinsky P, Morris RM, Seltzer JM, Storms WW, Wood RA. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics. 2000; 105:E23. PMID: 10654983.

Fig. 1
Coronal CT of the paranasal sinuses in a 7-year-old child showing extensive mucosal thickening within all paranasal sinuses, ostiomeatal complexes and nasal cavity.
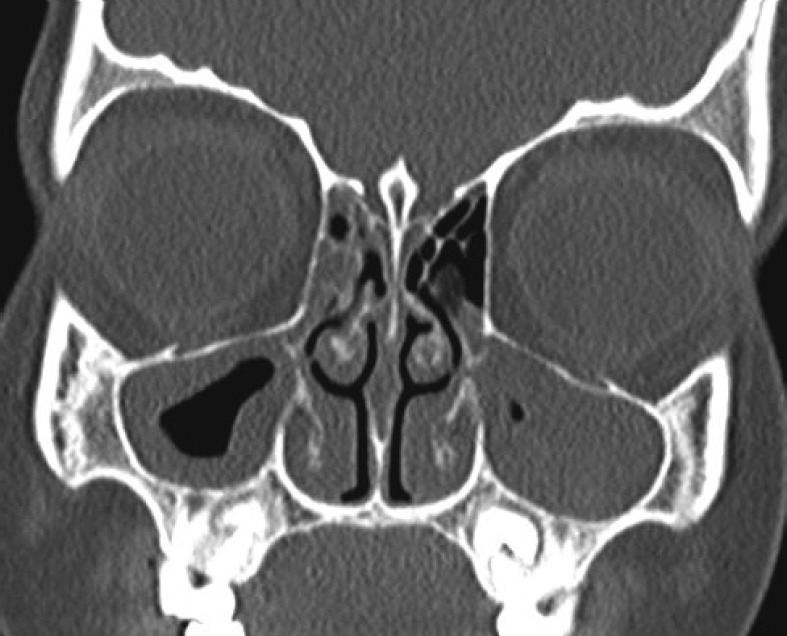
Table 1
Prevalence (%) of the symptoms of allergic rhinoconjunctivitis in children of Indian subcontinent, Asia-Pacific and the Oceanic countries during the ISAAC phases I and III
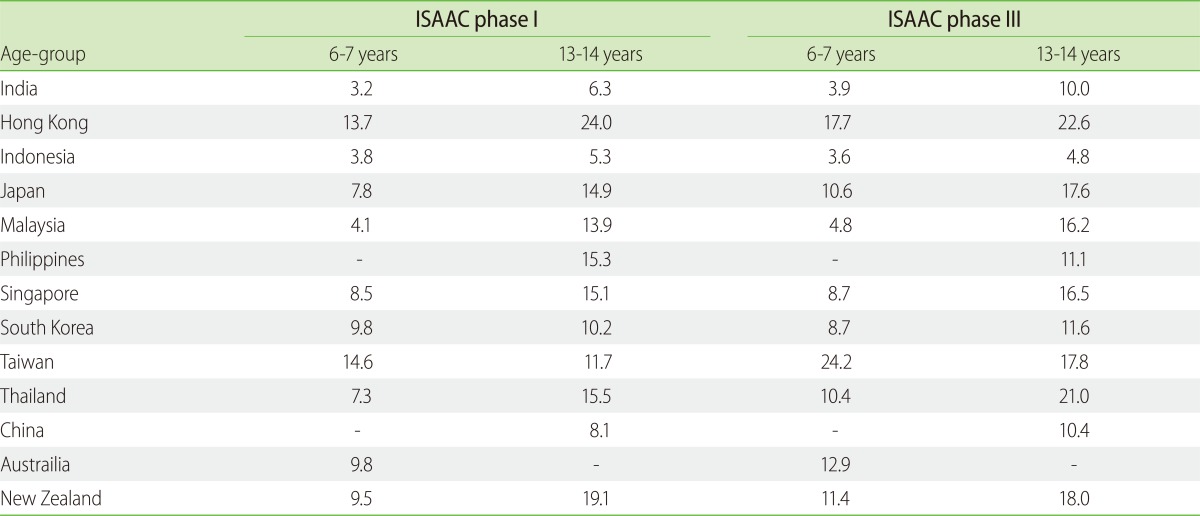
Adapted from reference [6]. ISAAC, International Study for Asthma and Allergies in Childhood.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download