Abstract
Background
Exacerbation of asthma has a negative impact on quality of life and increases the risk of fatal asthma. One of the known risk factors for patients with a history of near-fatal asthma is reduced sensitivity to dyspnea.
Objective
We aimed to identify patients with such risk before they experienced severe exacerbation of asthma.
Methods
We analyzed asthma symptoms and peak expiratory flow rate (PEFR) values of 53 patients recorded daily in a diary over a mean period of 274 days. Patients matched their symptoms to one of eight categories ranging in severity from 'absent' to 'severe attack'. We then analyzed the relationship between PEFR and asthma symptoms by dividing the PEFR value by the values of clinical parameters, including asthma symptom level.
Results
Average PEFR was 75.2% (50.5-100%) in the 'absent' symptom category, 64.5% (36.6-92.6%) in 'wheeze', 57.3% (25.0-94.7%) in 'mild attack' and 43.6% (20.4-83.1%) in 'moderate attack', with the personal best reading taken as 100%. Thus, differences in PEFR in patients in the same symptom category varied widely. PEFR in wheeze, mild attack and moderate attack did not correlate significantly with duration of asthma, forced expiratory volume in one second or proportion of personal best to standard predicted PEFR values. These PEFRs showed no significant difference in groups divided by type of regular treatment, but showed a significant negative correlation with the coefficient of variation (CV) of PEFR when asthma symptoms were absent. CV for absent symptoms should be between +4.0 and -4.0% when using regression analysis to measure PEFR if the decreased PEFR is in agreement with guidelines.
The Wright peak flow meter was introduced in 1958, but clinicians at that time did not recognize its value for the management of asthma [1]. Later, reports appeared which showed good correlation between peak expiratory flow rate (PEFR) and forced expiratory volume in one second (FEV1) [2-5] as well as good outcome when PEFR was utilized for the management of asthma [6-10]. Following the publication of recommended guidelines for asthma by the National Institutes of Health and the Japanese Society of Allergology [11-13], in which a moderate attack is defined as a PEFR between 60% and 80% of the personal best, PEFR monitoring has become popular in Japan because it is easily performed and is fairly accurate.
PEFR monitoring by keeping an asthma diary has two important uses. First, physicians can easily determine the level of asthma control since the patient's last visit because they can compare the new PEFR with that recorded in the asthma diary at the time of the previous visit. The diary also allows physicians to manage asthma objectively without patient input, which avoids insufficient treatment of asthma based on patients misperceiving and thus misreporting the severity of their asthma. Second, in relation to patients themselves, it is useful for those patients who do not always perceive their asthma symptoms accurately, especially those who misperceive their symptoms to be mild instead of more serious airway obstruction. This reduced sensitivity to dyspnea is known to be one of the risk factors of fatal asthma in patients with a history of near-fatal asthma [14-19]. Therefore, PEFR monitoring is useful for both patients and doctors.
By keeping an asthma diary, patients learn to perceive their asthma symptoms more accurately. This will ultimately assist them in evaluating their asthma symptoms more accurately without the need to check their PEFR. However, simply keeping a diary cannot improve asthma, because PEFR monitoring alone is unlikely to improve mortality [20]. Our impression is that patients who have frequent asthma attacks often misinterpret their symptoms as being mild, when in fact they may have serious airway obstruction. Therefore, it is important that patients with exacerbations of asthma analyze their asthma diaries in relation to PEFR monitoring so that they can understand the association between PEFR and their symptoms.
The aim of this study was to identify patients with reduced sensitivity to dyspnea before they experience severe exacerbation of asthma and require medical intervention. To identify these patients, we compared PEFRs recorded in the asthma diary with other clinical data.
We prospectively enrolled patients with asthma who recorded both asthma symptoms and PEFRs on a daily basis for an average period of 274 days. Then, the relationship between PEFRs and symptoms noted in the diaries was retrospectively analyzed. If the asthma diary included at least one record of an attack of dyspnea or wheezing in the previous month, the physician explained the purpose of the study to the prospective subject. If the patient consented to participate, he/she was enrolled and the physician was provided all of the asthma diaries kept by the patient (Fig. 1). Pulmonary diffusing capacity was measured and a chest X-ray taken to exclude patients with chronic obstructive pulmonary disease. The study protocol was approved by the ethics committee of Dokkyo Medical University (No 23059).
Fifty-three patients were enrolled in the study (Table 1). Forty-two patients regularly used inhaled corticosteroids (<400 µg/day of beclomethasone (BDP), n = 10; 400 µg/day of BDP, n = 17; >400 µg/day of BDP, n = 15), 33 regularly used oral corticosteroids, and 31 regularly used both. Asthma diaries, which were provided by these subjects, included data that covered periods ranging from 21 to 832 days. The mean duration of recording asthma data was 274 days.
Patients noted their asthma symptoms in the diary twice a day (morning and evening) before self-administering asthma drugs such as theophylline, beta2-adrenergic agonists, and corticosteroids [22, 23]. They classified their symptoms according to the criteria of the Japanese Society of Allergology as one of eight categories ranging in severity from 'absent' to 'severe attack' (Table 2). Subjects also measured PEFR twice a day (morning and evening) by using a peak flow meter before self-administering asthma drugs and noted the PEFR value in their asthma diary. The best PEFR for the duration of analysis was defined as the "personal best PEFR". There are three models of peak flow meters commercially available in Japan. Although we did not designate one specific model for study use, patients were asked to use the same peak flow meter throughout their observation period.
The average PEFR and standard deviation (SD) for each symptom category were calculated for each patient. Data are expressed as mean ± SD (minimum-maximum). To evaluate the variation of PEFR, the coefficient of variation (CV) was used. CV is calculated as SD/mean × 100 (%) and is useful for comparing variation between mean values [24]. Because mean PEFR is different between patents, CV is best for evaluating the variation of PEFR. CV of the PEFR for each symptom category was calculated for each patient.
We defined patients as having reduced sensitivity to dyspnea when they experienced a moderate attack with a PEFR of <60%. This is based on the definition of a moderate attack a PEFR between 60% and 80% of the personal best [25, 26]. When patients present asthma attacks, the sensitivity to dyspnea will be normal, if the decreased PEFR agrees with the above guideline. But, although the PEFR is decreased widely, if the patient feels as mild symptom, it will be insensitive for dyspnea.
The average PEFR was 43.6 ± 18.8% (20.4-83.1%) for 'moderate attack', 57.3 ± 18.2% (25.0-94.7%) for 'mild attack', 64.5 ± 13.5% (36.6-92.6%) for 'wheeze', 65.8 ± 15.8% (31.1-93.6%) for 'shortness of breath (SOB)', 55.8 ± 16.2% (32.5-79.2%) for 'severe cough', 67.5 ± 11.7% (31.1-93.6%) for 'mild cough', and 75.2 ± 11.1% (50.5-100%) for 'absent', with the personal best reading taken as 100% (Table 1). None of the patients experienced a severe attack during the observation period, although 24 patients presented <60% of personal best PEFR, which is classified as a severe attack according to the guidelines [25, 26]. Decreases in the PEFR varied widely in patients who experienced a moderate attack (20.4-83.1%), and some patients reported only mild symptoms despite having a very low PEFR.
Average PEFR for each patient who had experienced a moderate attack (n = 13), mild attack (n = 33) or wheeze (n = 52) was used to determine their sensitivity to dyspnea. Patients with lower PEFRs in each symptom category were evaluated for insensitivity to dyspnea.
First, PEFR was compared between patients divided into three groups according to type of regular asthma treatment: oral corticosteroids, inhaled corticosteroids including asthma drugs but without oral corticosteroids, and other treatments without inhaled or oral corticosteroids. No significant differences were observed between the groups (Fig. 2). Next, the correlation between average PEFR for each symptom severity category and pulmonary function was analyzed (Fig. 3). A significant correlation between PEFR and %V̇25 was observed for mild attack and wheeze. Moreover, average PEFR showed a significant negative correlation with CV for the absent symptom category (Fig. 4), but no significant correlation with the proportion of personal best to standard PEFR or the duration of asthma for any of the three symptom categories. The standard PEFR was obtained from a table of PEFR values for normal, healthy Japanese subjects [29].
Sensitivity to dyspnea was improved in some patients after their asthma was totally controlled over the course of the study, as a result of recording their symptoms and monitoring PEFR on a daily basis. The example of a 39-year-old female patient is shown in Fig. 5. PEFR was decreased to 30.6% in the first moderate attack, with an average PEFR of 77.3% and CV of 11% in the preceding month when asthma symptoms were recorded as being absent. When PEFR decreased to around 60%, she understood her symptoms to be SOB, and not moderate attack. However, when the patient experienced the next moderate attack after 3 months, PEFR had decreased to a lesser extent (67.3%), with a better average PEFR of 84.5% and CV of 6% in the preceding month when asthma symptoms were recorded as being absent. If she had experienced the same attack 3 months earlier, she might have interpreted the attack as SOB. The reason that she was able to understand her symptoms correctly was that her sensitivity to dyspnea had been improved. Thus, after asthma is totally controlled, sensitivity to dyspnea will improve.
In a moderate attack, PEFR should be between 60% and 80% of the personal best, and in a mild attack or wheeze, it should be greater than 80% of the personal best, as stipulated by the aforementioned guidelines [25, 26]. The regression lines in Fig. 4C show PEFR in a moderate attack (85.222 - 2.86466 × CV), mild attack (90.002 - 2.36989 × CV) and wheeze (86.617 - 1.67059 × CV). If the PEFR stipulated by the guidelines is ideal, then the CV for a moderate attack should be between 1.8% and 8.8%, and the CV for a mild attack and wheeze should be less than 4.2% and 4.0%, respectively. The variation in PEFR, when patients perceive their symptoms accurately according to the aforementioned guidelines, should be in agreement with the above three conditions, with a CV of 1.8-4.0%. CV should always be less than 4.0%. In the present study, CV, which is calculated by dividing SD by the mean, was used to avoid differences of mean PEFR values. Therefore, variation should be evaluated as ±CV instead of ±SD. On the basis of the above calculations, the variability of PEFR in the absence of asthma symptoms should be less than ±4.0%, and the range of variation should be <8.0% of the personal best. This suggests that, if the personal best PEFR value is 500, then the variability of PEFR while experiencing no symptoms should stay below 40.
It has been reported that patients with a history of near-fatal asthma show reduced sensitivity to dyspnea [14-19]. In other words, these patients incorrectly perceive their symptoms to be mild rather than to be a more serious case of airway obstruction, and as such they can experience a severe attack without premonitory symptoms. It is important, therefore, to determine which patients are at risk before they experience a severe attack. However, because it is not easy to test the sensitivity to dyspnea in every patient, we chose to analyze the PEFRs recorded in asthma diaries.
The majority of patients in our hospital with asthma are treated according to the guidelines and their condition is well controlled. In the present study, therefore, only 53 patients were enrolled. Furthermore, more than half of the patients were severe, because only those who presented with an attack of dyspnea or wheezing in the previous month were enrolled. The relationship between exacerbation of asthma and PEFR was analyzed, and the severity of asthma did have an effect on our study. We examined 13 patients with moderate attack, 33 with mild attack and 52 with wheeze over a long observation period, which was sufficient for analyzing factors to determine the risk of severe attack. Although we examined no patients with severe attack, 24 patients presented with <60% of the personal best PEFR, and those exacerbations should be regarded as severe attacks. In addition, it proved effective to evaluate the difference in PEFR for the same symptom category between patients. To analyze the insensitivity to dyspnea, we consider these subjects were useful in our study.
Before we analyzed the data, we expected that patients with reduced sensitivity to dyspnea would be categorized as having severe asthma because, in our clinical experience, asthma attacks are more prevalent in patients with severe asthma than mild asthma. However, the severity value divided by the treatment value did not show a significant relation to reduced sensitivity, and some patients showed normal sensitivity to dyspnea even when fully treated (including those treated with oral corticosteroids). Taking multiple drugs including oral corticosteroids is not a direct risk factor for reduced sensitivity to dyspnea in itself, but it is important that asthma is completely absent.
Airway remodeling is indicated by chronic and irreversible airway obstruction [30] and causes airway hyper-responsiveness, and recurrence of airway inflammation occurs readily [31]. Airway remodeling is a known risk factor for fatal asthma [32], and small airways are major sites of remodeling in fatal asthma [33]. V̇25 is an indicator of airway obstruction in small airways and is not easily improved by treatment. In the present study, significant correlation was seen between %V̇25 and PEFR in mild attack and wheeze. For moderate attack, a significant correlation is probable if a higher number of subjects are studied. Thus, airway remodeling may contribute to reduced sensitivity to dyspnea.
It has been reported that fluctuation in PEFR is related to airway hyper-responsiveness and is one of the risk factors for fatal asthma [34, 35]. It would be useful if airway hyper-responsiveness could be reliably predicted by the variation in PEFR without examination. This was the reason behind analyzing the relationship between CV of PEFR in the reported absence of asthma symptoms and initial airway hyper-responsiveness. However, no significant correlation was found (data not shown), because our results were based on a long period of observation. In Fig. 5A, a wide fluctuation of PEFR is expected to indicate an increase in airway hyper-responsiveness. On the other hand, a narrow fluctuation of PEFR is expected to indicate a decrease in airway hyper-responsiveness Fig. 5B. Because airway hyper-responsiveness is improved by treatment, the relationship between initial airway hyper-responsiveness and fluctuation in PEFR on a long period of observation cannot be established.
CV is suitable for evaluating the fluctuation in PEFR because differences in age, height and sex between patients are not confounding factors. When asthma symptoms are reportedly absent, patients' PEFRs should approximately match their personal best, and CV will be almost zero. However, patients with poorly controlled asthma showed fluctuations in PEFR in the reported absence of asthma symptoms. Although CV in this situation is a useful measure, it has two disadvantages. First, patients can become tired of calculating it on every occasion. Second, it is unclear why variation is better for totally controlled asthma. To resolve these problems, using the regression analysis data shown in Fig. 4C, we calculated PEFR variability for those patients who reported an absence of symptoms. The results showed that the PEFR variability in such patients needs to be below 8% to achieve the established relationship between PEFR and asthma symptoms according to the guidelines [25, 26]. Whether SD of ± 1 is an appropriate range for the variability is debatable; however, to accord with the guidelines, it is advisable to keep the PEFR variability of a patient who reports an absence of symptoms below 8%.
It is important to evaluate fluctuations in PEFR in the reported absence of asthma symptoms in patients without a family doctor, as suggested by Sugiyama et al [36]. They reported that patients who visited an emergency room with exacerbation of asthma could be roughly divided into two groups. One group comprised patients with severe asthma who were generally older: these patients often experience exacerbation of asthma despite being treated at hospital regularly. The other group comprised patients without a family doctor: although these patients believe their asthma to be mild, some of them have more than mild asthma. Asthma specialists do not always have the chance to evaluate patients' asthma because they visit emergency rooms only for exacerbation of the condition. If they are given a peak flow meter to take home in the emergency room, and if it is explained that a fluctuating or low PEFR value is not mild asthma, this may decrease the number of patients at risk of fatal asthma.
In conclusion, asthma diaries with PEFRs recorded contain important information. A peak flow meter is cheap and easy for patients to use and can easily be introduced into the patient's treatment by a physician who is not an asthma specialist. To ascertain which patients have reduced sensitivity to dyspnea, the most important factor to consider is the CV of PEFR when patients report an absence of asthma symptoms. When such patients present with fluctuating PEFR values, we should intervene in their treatment even when they claim to be stable.
Figures and Tables
Fig. 1
Flow chart for enrollment. COPD, chronic obstructive pulmonary disease; PEFR, peak expiratory flow rate.

Fig. 2
For each patient, the average value of peak expiratory flow rate (PEFR) was calculated for moderate attack, mild attack, and wheeze, in relation to personal best as 100%. Patients were divided into groups according to their regular treatment. The relationship between PEFR and regular treatment showed no significant relationship with reduced sensitivity to dyspnea for any of the symptoms of moderate attack, mild attack or wheeze. Data are expressed as mean ± SD. ICS, inhaled corticosteroid; N.S., not significant.

Fig. 3
For each patient, the average value of peak expiratory flow rate (PEFR) was calculated for moderate attack, mild attack, and wheeze, in relation to personal best as 100%. Correlations between PEFR and pulmonary function were analyzed for each symptom. A significant correlation was seen only for % flow rate at 25% of FVC (V̇25) in the case of mild attack and wheeze. PEFR for each symptom, which is stipulated by the aforementioned guidelines, is shown as a gray line. FVC, forced vital capacity; FEV1, forced expiratory volume in one second.

Fig. 4
For each patient, the average value of peak expiratory flow rate (PEFR) was calculated for moderate attack, mild attack, and wheeze, in relation to personal best as 100%. Neither the proportion of personal best to standard PEFR (A) nor the duration of asthma (B) showed a significant correlation with PEFR. However, the coefficient of variation (CV) of PEFR when asthma symptoms were reported to be absent showed a significant negative correlation with PEFR (C). PEFR for each symptom, which is stipulated by the aforementioned guidelines, is shown as a gray line. N.S., not significant.

Fig. 5
Peak expiratory flow rate (PEFR) monitoring for a 39-year-old female patient as an example case. PEFR was decreased to 30.6% in first moderate attack, and average PEFR and coefficient of variation (CV) in the reported absence of asthma symptoms for 1 month before the attack were 77.3% and 0.11, respectively (A). However, when the patient presented the next moderate attack 3 months after her asthma was fully controlled after episode A, PEFR was much less decreased to 67.3%, which the patient presented as shortness of breath (SOB) in episode A. Average PEFR and CV in the absence of asthma for the preceding 1 month were also better at 84.5% and 0.06, respectively (B). Thus, reduced sensitivity to dyspnea was improved.

Table 1
Baseline clinical characteristics
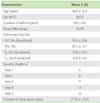
FVC, forced vital capacity; FEV1, forced expiratory volume in one second; V̇50, flow rate at 50% of FVC; V̇25, flow rate at 25% of FVC. *GINA 2010 [21].
ACKNOWLEDGMENTS
The authors thank Dr. Shinji Motojima for statistical advice and Miss Ayano Takeda for assistance with analyses.
References
1. Gregg I. Can measurement of peak expiratory flow enhance compliance in chronic asthma? Eur Respir J. 1992. 5:136–138.
2. Rosenblatt G, Alkalay I, McCann PD, Stein M. The correlation of peak flow rate with maximal expiratory flow rate, one-second forced expiratory volume, and maximal breathing capacity. Am Rev Respir Dis. 1963. 87:589–591.
3. Fairbairn AS, Fletcher CM, Tinker CM, Wood CH. A comparison of spirometric and peak expiratory flow measurements in men with and without chronic bronchitis. Thorax. 1962. 17:168–174.

4. Troyanov S, Ghezzo H, Cartier A, Malo JL. Comparison of circadian variations using FEV1 and peak expiratory flow rates among normal and asthmatic subjects. Thorax. 1994. 49:775–780.
5. Gautrin D, D'Aquino LC, Gagnon G, Malo JL, Cartier A. Comparison between peak expiratory flow rates (PEFR) and FEV1 in the monitoring of asthmatic subjects at an outpatient clinic. Chest. 1994. 106:1419–1426.
6. Boutin C, Barré A, Charpin J. Comparison between peak expiratory flow rate and daily report of the symptoms in asthmatic children. Bull Physiopathol Respir (Nancy). 1975. 11:285–294.
7. Pauli G, Bigot H, Pelletier A, Kopferschmitt-Kubler MC, Roegel E. A study of the criteria used for clinical evaluation of prophylactic treatment in bronchial asthma. Eur J Respir Dis. 1985. 67:369–377.

8. Malo JL, L'Archevêque J, Trudeau C, d'Aquino C, Cartier A. Should we monitor peak expiratory flow rates or record symptoms with a simple diary in the management of asthma? J Allergy Clin Immunol. 1993. 91:702–709.

9. Gern JE, Eggleston PA, Schuberth KC, Eney ND, Goldstein EO, Weiss ME, Adkinson NF Jr. Peak flow variation in childhood asthma: a three-year analysis. J Allergy Clin Immunol. 1994. 93:706–716.

10. Motojima S, Kurusu H, Fukuda T, Makino S. Management of asthma in use of PEF. Complication with symptom. Ther Res. 1993. 14:3053–3061.
11. Expert Panel on the Management of Asthma. Guidelines for the Diagnosis and Management of Asthma: Expert Panel Report. 1991. Bethesda: National Asthma Educational Program, Office of Prevention, Education, and Control, National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, Public Health Service.
12. Asthma Committee of Japanese Society of Allergology. Makino S, editor. Guidelines for diagnosis and management of bronchial asthma. Guidelines for the management of allergic diseases. 1993. Tokyo: Life Science Medica;1–37.
13. Japanese Society of Allergology. Committee on the Definition, Treatment, and Management of Bronchial Asthma. Guidelines for the diagnosis and management of bronchial asthma. Allergy. 1995. 50:27 Suppl. 1–42.
16. Molfino NA, Nannini LJ, Martelli AN, Slutsky AS. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991. 324:285–288.

18. Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. N Engl J Med. 1994. 330:1329–1334.

19. Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002. 121:329–333.

20. Grampian Asthma Study of Integrated Care (GRASSIC). Effectiveness of routine self monitoring of peak flow in patients with asthma. BMJ. 1994. 308:564–567.
21. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2010. Treatment to achieve control. 2010. Geneva: GINA;61–65.
22. Yamamura Y, Mitsui S, Sudo M, Takishima T, Kobayashi S, Nemoto T, et al. Classification of severity of asthma according to Japanese Society of Allergology (in Japanese). Jpn J Allergol. 1983. 32:1186–1199.
23. Miyamoto T, Shida T, Tomioka H, Makino S, Kabe J, Nakajima S, et al. Classification of severity of asthma according to Japanese Society of Allergology (in Japanese). Jpn J Allergol. 1994. 43:71–80.
24. Pagano M, Gauvreau K. Principles of biostatistics. 2000. 2nd ed. Duxbury: Pacific Grove.
25. The Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2009. 2009. Bethesda: National Institutes of Health publications.
26. Japanese Society of Allergology. Japanese Guidelines for the Diagnosis and Treatment of Allergic Diseases 2010. 2010. Tokyo: Kyowa Kikaku Tsushin.
27. Baldwin ED, Cournand A, Richards DW Jr. Pulmonary insufficiency; physiological classification, clinical methods of analysis, standard values in normal subjects. Medicine (Baltimore). 1948. 27:243–278.
28. Cherniack RM, Raber MB. Normal standards for ventilatory function using an automated wedge spirometer. Am Rev Respir Dis. 1972. 106:38–46.
29. Miyamoto T, Makino S, Toda M, Tsukioka K. Peak expiratory flow in normal, healthy Japanese subjects. 1995. Tokyo: Kyowa Kikaku Tsushin.
30. James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989. 139:242–246.

31. Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J. 1998. 12:1252–1256.

33. Dolhnikoff M, da Silva LF, de Araujo BB, Gomes HA, Fernezlian S, Mulder A, Lindeman JH, Mauad T. The outer wall of small airways is a major site of remodeling in fatal asthma. J Allergy Clin Immunol. 2009. 123:1090–1097. 1097.e1

34. Brand PL, Postma DS, Kerstjens HA, Koëter GH. The Dutch CNSLD Study Group. Relationship of airway hyperresponsiveness to respiratory symptoms and diurnal peak flow variation in patients with obstructive lung disease. Am Rev Respir Dis. 1991. 143:916–921.

35. Hetzel MR, Clark TJ, Branthwaite MA. Asthma: analysis of sudden deaths and ventilatory arrests in hospital. Br Med J. 1977. 1:808–811.

36. Sugiyama K, Yamada I, Ohara T, Tatewaki M, Hayashi Y, Arai R, Kamiya K, Fukushima F, Hirata H, Arima M, Fukushima Y, Fukuda T. An analysis of characteristics of patients with exacerbation of asthma in a large university hospital in Japan. Asian Pac J Allergy Immunol. 2010. 28:242–249.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download