This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
To achieve pulp-dentin complex regeneration with tissue engineering, treatment efficacies and safeties should be evaluated using in vivo orthotopic transplantation in a sufficient number of animals. Mice have been a species of choice in which to study stem cell biology in mammals. However, most pulp-dentin complex regeneration studies have used large animals because the mouse tooth is too small. The purpose of this study was to demonstrate the utility of the mouse tooth as a transplantation model for pulp-dentin complex regeneration research.
Materials and Methods
Experiments were performed using 7-week-old male Institute of Cancer Research (ICR) mice; a total of 35 mice had their pulp exposed, and 5 mice each were sacrificed at 1, 2, 4, 7, 9, 12 and 14 days after pulp exposure. After decalcification in 5% ethylenediaminetetraacetic acid, the samples were embedded and cut with a microtome and then stained with hematoxylin and eosin. Slides were observed under a high-magnification light microscope.
Results
Until 1 week postoperatively, the tissue below the pulp chamber orifice appeared normal. The remaining coronal portion of the pulp tissue was inflammatory and necrotic. After 1 week postoperatively, inflammation and necrosis were apparent in the root canals inferior to the orifices. The specimens obtained after experimental day 14 showed necrosis of all tissue in the root canals.
Conclusions
This study could provide opportunities for researchers performing in vivo orthotopic transplantation experiments with mice.
Keywords: Mouse, Pulpitis, Dental pulp necrosis, Regeneration, Stem cells
INTRODUCTION
The conventional treatment modality for teeth with an irreversibly damaged or necrotic dental pulp is root canal therapy, which involves complete removal of the damaged tissue and tight sealing of the root canal space with a synthetic material [
1]. However, this treatment does not regenerate the pulp-dentin complex but replaces. Therefore, many researchers have attempted to regenerate the pulp-dentin complex through tissue engineering.
To achieve pulp-dentin complex regeneration with tissue engineering, appropriate candidate substances have been proposed and tested in animal models [
234]. Unlike an
in vitro environment in which several factors can be easily controlled,
in vivo experiments with animal teeth require particularly advanced skills and techniques. Because of these difficulties,
in vivo studies on pulp-dentin complex regeneration to date have usually involved ectopic transplantation of the candidate substance into the subcutaneous tissue or renal capsule rather than orthotopic transplantation directly into the teeth [
5]. Only several studies have been performed the orthotopic transplantation of a candidate substance in large animals such as dogs, pigs, ferrets, and monkeys [
678]. However, before applying these candidate substances in clinical trials, their treatment efficacies and safeties should be evaluated using
in vivo orthotopic transplantation in a sufficient number of animals. Experiments using sufficient numbers of animals are restricted by breeding, costs and ethical issues involved in securing a sufficient number of experimental animals. In contrast, mice are relatively inexpensive, reproduce quickly, and can be easily manipulated genetically. Despite these advantages of mice, most pulp-dentin complex regeneration studies have used large animals because the mouse tooth, of which the diameter is only 1.5–2 mm, has been considered too small.
Understanding how pulpitis develops over time after pulp exposure to bacteria is essential in creating a transplantation model. Until now, traditional instrumentation has not been suitable for cavity preparation and studies on the progression of pulpitis in mice have been limited. However, recently developed surgical operating microscopes provide magnification and illumination and elaborate instruments such as the micro bur allow for a more precise procedure. The purpose of this preliminary study was to demonstrate the utility of the mouse tooth in a transplantation model for pulp-dentin complex regeneration research.
MATERIALS AND METHODS
Animals
Experiments were performed using 7-week-old male Institute of Cancer Research (ICR) mice (30–35 g) supplied by Orient Bio, Inc. (Seungnam, Korea). ICR mice have a pair of incisors and three pairs upper and lower molars. Molars are permanently rooted while the incisors have an open root and grown continuously. Animals were housed in individually ventilated caging under sanitary conditions in light (12 hours on, 12 hours off). The temperature was 23°C ± 1°C and the humidity was 50% ± 5%. The mice were fed with irradiated pellet food ad libitum and had free access to sterilized drinking water. The cage bottoms and drinking bottles were changed weekly. The mice were allowed to acclimatize for at least one week prior to the experiments. All animal experiments were performed in accordance with the Guideline for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Yonsei University.
Experimental group design and animal experimentation
To verify the appearance of inflammation progression over time, a total of 35 mice had their pulp exposed, and five mice each were sacrificed at 1, 2, 4, 7, 9, 12 and 14 days after pulp exposure. As a control group to check normal pulp status, unprepared teeth were used. All procedures were performed under magnification (×20) with a surgical operating microscope (Global Surgical, St. Louis, MO, USA) except for anesthesia. Following anesthesia by an intraperitoneal injection of a combination of zolazepam and tiletamine as Zoletil 50
® (30 mg/kg, Virbac, Carros, France) and xylazine as Rompun
® (10 mg/kg, Bayer, Leverkusen, Germany), the mice were fixed with wire and elastic (
Figure 1A), a cavity was prepared with a 0.5 mm diameter carbide bur (diameter, 0.5 mm) (H1.FG.005; Komet, Gebr Brasseler GnbH & Co KG, Lemgo, Germany) on the occlusal aspect of the maxillary first right molar in the center of the tooth according to the mesiodistal plane until the pulp was exposed. After pinpoint pulp exposure, the access opening was subsequently enlarged mechanically using sizes 15 and 20 endodontic hand files (K-file
®, Dentsply Maillefer, Ballaigues, Switzerland) to control the pulp exposure size to approximately 200 µm (the size of the tip of the K-file) (
Figure 1B). The cavity was not sealed to maintain bacterial invasion into the dental pulp.
Figure 1
The clinical procedure used for pulp exposure. (A) After anesthesia by an intraperitoneal injection of Zoletil 50® and Rompun®, the mouse is fixed with wire and elastic. (B) After preparing a cavity with a bur, the pulp is exposed using sizes 15 and 20 endodontic hand files.

Histology
Animals were sacrificed at various durations according to the experimental design. Under deep anesthesia using a combination of zolazepam and tiletamine as Zoletil 50® (30 mg/kg) and xylazine as Rompun® (10 mg/kg), animals were sacrificed by 100% carbon dioxide inhalation. Following removal of most of the soft tissues, the maxillae of the mice were immersed in 4% paraformaldehyde for 24 hours at 4°C. The maxillae of the mice were dissected, rinsed in phosphate buffered saline (PBS) for 60 minutes and decalcified for 4 weeks in 5% ethylenediaminetetraacetic acid (EDTA) with 4% sucrose in PBS with a pH of 7.4 and agitation at room temperature. The solution was renewed every week. After decalcification, samples were dehydrated through increasing grades of isopropyl alcohol. The dehydration process consisted of a series of isopropyl alcohol rinses starting with a 70% solution for 1 hour and followed by 80%, 90%, 95%, and 100% solutions for 1 hour. After dehydration, samples were embedded in paraffin blocks according to standard procedures. After being embedded in paraffin, the samples were cut with a microtome (Leica, Berlin, Germany) in 6-µm sections and then stained with hematoxylin and eosin. Slides were observed under high magnifications of 40× to 400× by light microscopy (Nikon, Tokyo, Japan).
RESULTS
In the negative control group, the histology of the pulp appeared normal. Loose connective tissue composed of fibroblasts and blood vessels formed the dental pulp located in the dental cavity. Odontoblasts formed a layer lining the periphery of the dental pulp and had a process extending into the dentin (
Figure 2).
Figure 2
Normal pulp. (A) Fibroblasts, blood vessels and loose connective tissue form the dental pulp located in the dental cavity (×40). (B) Odontoblasts form a layer lining the periphery of the dental pulp and have a process extending into the dentin (×100).
PDL, periodontal ligament.

In the experimental group, until 1 week postoperatively, the tissue below the pulp chamber orifice appeared normal. The remaining coronal portion of the pulp tissue was inflammatory and necrotic (
Figure 3). The specimens obtained after experimental day 1 showed hyperemia. Dilation of the pulpal blood vessels was observed, and large numbers of red blood cells were seen around the fibroblasts. At postoperative day 2, the specimens showed a pattern of inflammation with deposition of inflammatory cells such as neutrophils and lymphocytes in the pulp chamber superior to the orifices. At postoperative day 4, the pulp chamber showed a microabscess in the distal pulp horn and a severe accumulation of chronic inflammatory cells was observed in the mesial pulp horn. At postoperative day 7, specimens showed well-defined caseous necrosis surrounded by chronic inflammatory cells in the center of the pulp chamber (
Figure 3) and inflammation and necrosis were apparent in the root canals inferior to the orifices. In specimens on postoperative day 9, all tissue above the orifices showed necrosis, while inflammatory cells and normal tissue were observed inferior to the orifices (
Figure 4). The specimens on postoperative day 12 showed necrosis up to one-third of the distance to the apex. The specimens obtained after experimental day 14 showed necrosis of all tissue in the root canals and inflammatory cells were observed inferior to the apex (
Figure 5).
Figure 3
Seven days after pulp exposure. (A) Low magnification (×40) and (B) high magnification (×200). Well-defined caseous necrosis surrounded by chronic inflammatory cells is observed in the center of the pulp chamber.
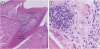
Figure 4
Nine days after pulp exposure. (A) Low magnification (×40) and (B) high magnification (×100). All tissues above the orifices show necrosis, while inflammatory cells and normal tissues are observed inferior to the orifices.

Figure 5
(A) The specimens obtained after 14 experimental days show necrosis of all tissue in the root canal (×40). (B, C) At higher magnification, inflammatory cells are observed inferior to the apex (×100, ×200).

DISCUSSION
The dental pulp may be exposed to a number of irritants that are noxious to the health of the pulp. Bacterial infection due to pulp exposure is the most common cause of pulp disease [
9]. Thus, in order to create a mouse model, the pulp was exposed as a method of inducing pulpitis and pulp necrosis. Although there are many studies that describe pulp pathology from a clinical perspective, few studies have concentrated on the relationship between bacterial invasion and the histopathological substrate specific to the inflammatory process developed in the pulp [
1011].
The pulp status was observed histologically for 14 days after pulp exposure. Until 7 days, inflammation was restricted to above the orifice level. By 14 days after pulp exposure, necrosis had progressed as far as the root apex, and all cells and tissue within the root canals were found to have undergone necrosis. These results are consistent with previous studies reporting that when dental pulp is exposed to bacteria due to caries or trauma, infection and pulp necrosis start at the exposure site and progress gradually in an apical direction [
12].
Looking at specimens obtained more than 1 day after pulp exposure, hyperemia and red blood cells were observed throughout the pulp from the pulp horn to the root apex. This sort of vasodilation is a feature of acute inflammation. Acute inflammation is characterized by marked vascular changes, increased permeability and increased blood flow, which are induced by the actions of various inflammatory mediators. The first vascular reaction during acute inflammation is vasodilation, which may increase the blood volume in the inflamed area [
13]. In specimens from postoperative days 2–4, inflammation was observed in the pulp chamber superior to the orifices. A high concentration of chronic inflammatory cells, such as lymphocytes and macrophages, and a microabscess were observed in the pulp horn. Specimens from 7 days postoperatively showed that a considerable part of the pulp in the middle chamber underwent caseous necrosis. Nevertheless, there was a distinct transition to the surrounding relatively normal pulp tissue. A clear demarcation line could be observed between the tissue disorganized by inflammatory infiltration and the surrounding normal tissue. Within the first week after pulp exposure, necrosis and inflammatory cell accumulation were restricted to above the orifices and the pulp in the root canals remained in a normal state. Prior to advances in pulp biology, it was believed that pulp inflammation could not be reversed once it had started and that it would inevitably progress to apoptosis and pulp necrosis [
14]. However, advances in pulp biology combined with clinical evidence in treating deep caries have started to modify the traditional view of pulp inflammation [
91516]. It is thought that this result could be useful in not only pulp-dentin complex regeneration research but also in vital pulp therapy such as partial pulpotomy.
One week after pulp exposure, inflammation and necrosis progressed inferior to the orifices into the root canals; after 2 weeks, all the tissue within the root canals had undergone necrosis and inflammatory cells were observed inferior to the apex. If a blood supply and removal of bacteria are considered essential to cell survival, once 14 or more days have passed since pulp exposure, appropriate candidate transplantation would have to be attempted after removing all the tissue within the root canal as far as the apex.
The present study has demonstrated the feasibility of using the mouse in an animal model for research into pulp-dentin complex regeneration. The mouse tooth is small and difficult to access; therefore, it has not been used in dental pulp research to date. However, using a dental operating microscope and micro-instruments can overcome these limitations.
A limitation of this study is that it was difficult to produce slides, so the progression of inflammation could not be analyzed statistically. This is a technical problem that can be overcome through more experimentation.
This study could provide opportunities for researchers investigating in vivo orthotopic transplantation in mice. Based on this study, if a mouse tooth transplantation model is continuously developed, it will be very helpful in dental pulp research development. Moreover, the use of mice would also have the effect of reducing the numbers of large animals sacrificed in experiments.
CONCLUSIONS
Up to seven days after exposure of the dental pulp in mice, inflammation and necrosis was limited to the area superior to the orifice level. By 14 days after pulp exposure, necrosis had progressed as far as the root apices, and all the cells and tissues in the root canals had undergone necrosis.






 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download