Abstract
Phlegmonous gastritis is a rare and rapidly progressive bacterial infection of the stomach wall, with a high mortality rate. Antibiotics with or without surgical treatment are required for treatment. We present a case in which phlegmonous gastritis occurred during the diagnostic evaluation of early gastric cancer. The patient showed improvement after antibiotic treatment, but attempted endoscopic submucosal dissection failed because of submucosal pus. We immediately applied argon plasma coagulation since surgical resection was also considered a high-risk procedure because of the submucosal pus and multiple comorbidities. However, there was local recurrence two years later, and the patient underwent subtotal gastrectomy with lymph node dissection. Considering the risk of incomplete treatment immediately after recovery from phlegmonous gastritis and that recurrent disease can be more difficult to manage, delaying treatment and evaluation until after complete recovery of PG might be a better option in this particular clinical situation.
Phlegmonous gastritis (PG) is an uncommon and fatal disease characterized by suppurative bacterial infection of the stomach wall.12 It can progress rapidly and even form grossly suppurative exudate or pus within several days. Thus, strong clinical suspicion and rapid diagnosis are important to the prognosis. With antibiotic therapy, the mortality rate has decreased. However, the overall mortality is still about 27%.3
Although there are several reports about PG, to the best of our knowledge, there has been no report of its development during the diagnostic evaluation and following treatment of early gastric cancer (EGC) after diagnosis of PG. Herein, we report a case of PG that developed after performing a biopsy for the diagnosis of EGC. The patient had multiple comorbidities of alcoholic liver cirrhosis, diabetes mellitus, hypertension, and a history of right posterior liver sectionectomy for hepatocellular carcinoma. After successful treatment of PG with antibiotics, endoscopic ablation therapy (argon plasma coagulation, APC) was performed for the treatment of gastric cancer. However, local recurrence was found after two years, and the patient underwent subtotal gastrectomy with lymph node dissection.
A 74-year-old man with alcoholic liver cirrhosis underwent esophagogastroduodenoscopy (EGD) for varix evaluation. The patient had diabetes mellitus, hypertension, and alcoholic liver cirrhosis with Child-Pugh class A liver function. He had undergone right posterior sectionectomy for hepatocellular carcinoma 5 years prior. On EGD, two lesions were noted: a 1.2-cm ill-defined erythematous depression at the distal antrum and a 0.5-cm erythematous mucosal area at the proximal antrum (Fig. 1A, B). Biopsy specimens were obtained from the two lesions. Because of continuous bleeding at the proximal antrum after biopsy, endoscopic saline was administered and the bleeding stopped.
Nine hours later, he visited the emergency department of a local hospital because of upper abdominal pain and vomiting and was transferred to our hospital. Blood pressure was 141/71 mmHg and the heart rate was 99 beats per minute; his body temperature was 36.7℃. Bowel sounds were normoactive, and the upper abdomen was tender to palpation, but there was no evidence of peritonitis.
The blood test showed leukocytosis with neutrophilic left shift (white blood cell count, 18,490/µl, 88.5% neutrophils). Serum Creactive protein level was elevated at 28.68 mg/dl. The patient did not have anemia, and serum alanine aminotransferase, total bilirubin, alkaline phosphatase, lactate dehydrogenase, amylase, and lipase were within normal ranges. Abdominal computed tomography (CT) showed marked edematous wall-thickening in the gastric antrum, with an air bubble. However, no evidence of pneumoperitoneum was found (Fig. 1C). EGD performed on the same day showed diffuse thickening of mucosal folds, which was compatible with PG and biopsy-induced mucosal erosion (Fig. 1D, E). No evidence of bleeding or perforation was found.
No organism was cultured from his blood sample. The epigastric pain gradually disappeared after antibiotic treatment with ceftriaxone 2 g/day. Histologic results of the biopsies showed well-differentiated adenocarcinoma at the distal antrum and chronic gastritis at the proximal antrum.
After 9 days of hospitalization, we tried to perform endoscopic submucosal dissection (ESD) for EGC. However, the attempt failed, because the stomach was still diffusely edematous and purulent discharge made the procedure difficult (Fig. 2A). ESD seemed to be impossible in the near future because of submucosal pus and subsequent fibrosis. In addition, surgical resection such as subtotal gastrectomy was considered a high-risk procedure because the stomach wall was filled with pus and the patient had multiple comorbidities. Thus, we immediately applied APC for treatment of EGC (Fig. 2B). APC was performed as previously described.4 The equipment for APC included an argon gas source (APC300; Erbe Elektromedizin, Tübingen, Germany) and high-frequency generator (VIO 300D; Erbe Elektromedizin). The argon gas flow rate was 2.0 L/min, with the current set at 60 W. Culture of the purulent discharge was not attempted.
The EGD at 14 days after APC still showed a whitish purulent discharge but improved mucosal edema. Three months after APC, EGD showed APC scars without discharge or edematous mucosa (Fig. 3A). A biopsy was performed and no evidence of adenocarcinoma was found. Six months after APC, EGD biopsy showed adenocarcinoma in the APC scars and we repeated the APC (Fig. 3B). At the 9-, 12-, and 18-month follow-ups after the initial APC, EGD biopsy showed chronic gastritis. At the 24-month follow-up after initial APC, adenocarcinoma was again found in the APC scars (Fig. 3C). Thus, we decided to perform subtotal gastrectomy with lymph node dissection (D1 plus alpha) for recurrent EGC. The operation was successful without complications. Final pathology revealed a 2.2-cm EGC with tubular, well differentiated adenocarcinoma confined within the muscularis mucosa. No metastasis was detected among 16 harvested lymph nodes. The patient has not shown any recurrence in the subsequent 13 months.
PG is a rare disease, reported for the first time in 1862. About 500 cases have been published worldwide, with 15 cases in Korea.356 PG is caused by bacterial infection spreading through the stomach wall. The inflammation invades the submucosa or muscle layer and creates a suppurative abscess and infectious emphysema of the gastric wall. It can even spread to the esophagus or entire gastrointestinal tract.7
The most common symptom is abdominal pain (95%), followed by nausea or vomiting (68%), fever (57%), and hematemesis (14%).89 The progression is rapid, and even sepsis was reported at diagnosis.10 The most common pathogen is Streptococcus spp. (57%) but causative organisms can include Enterococcus spp., Klebsiella pneumoniae, Staphylococcus spp., Haemophilus influenzae, Proteus, and clostridia.311 Mucormycosis has been reported as an etiologic agent.
The main predisposing factor for PG is an immunocompromised state including alcoholism, diabetes mellitus, human immunodeficiency virus infection, chronic hepatitis, and achlorhydria.81213 PG has also been reported after endoscopic procedures like ESD,14 endoscopic ultrasound-guided fine-needle aspiration,15 and endoscopic mucosal resection.16 In this report, we presented a case with PG after biopsy for the diagnosis of EGC. The patient had comorbidities of alcoholic liver cirrhosis, diabetes mellitus, hypertension, and a history of liver resection for hepatocellular carcinoma. In a previous report, Kim et al.6 reported that 8 of 11 Korean patients had underlying conditions.
PG can be diagnosed by endoscopy with culture of the purulent discharge, endoscopic ultrasound, and CT scan.1718 Endoscopic findings can show gastric wall edema, purulent exudate, or gangrene. CT or ultrasound shows thickening of the gastric wall, abscess, or an air bubble in the gastric wall. In our case, the purulent discharge, edematous gastric wall, and air bubble were seen on EGD and CT at diagnosis. The edematous gastric wall improved, but a whitish purulent discharge was still present 23 days after diagnosis. Purulent discharge improved on follow-up EGD 3 months after initial diagnosis.
The mortality of PG was previously reported to be 42% to 67%. More recent data, however, indicate that the mortality has been decreasing; for example, Rada-Palomino et al.3 reported 27% (12/45) mortality in 2014. Predisposing conditions were suggested as a factor affecting mortality. Patients with predisposing conditions showed higher mortality (33%) than patients without predisposing conditions (17%). In addition, early recognition of the condition and prompt treatment lead to better survival.1
Antimicrobial agents are a mainstay of treatment, with surgical resection of infected tissue when necessary. The diversity of causative organisms and the natural history of the disease make it crucial to urgently initiate empirical broad-spectrum antimicrobial therapy. It is not feasible to compare the results of medical treatment with surgery because available data are based on case series that are outdated. There is a broad range of reported postoperative mortality (18%~75%), as well as of response to medical treatment (0%~100%).16811 In Korea, 10 reports with 15 cases of PG have been published.5619 The mortality rates for surgically or medically treated patients were 44.4% (4/9) and 0% (0/6), respectively. The difference in mortality rates could be because of a milder disease state in medically-treated cases, but this is uncertain. If the disease is diagnosed at an early phase and is localized, it can be treated with antibiotics and supportive management.
To our knowledge, the present case is the first report of treatment of EGC after diagnosis of PG. The optimal time to treat gastric cancer in patients with PG is unknown. Immediate surgical resection could treat gastric cancer and PG simultaneously, but previous surgical treatments of PG have shown high mortality rates.5619 Our case showed that endoscopic treatment (ESD or APC) for EGC immediately after recovery from PG has the risk of incomplete treatment. Because recurrent disease can be more difficult to manage, delaying treatment and evaluation of the gastric cancer until after full recovery from PG might be a better option in this particular clinical situation.
In summary, PG developed after endoscopic biopsy in a patient with several comorbidities. After improvement of PG with antibiotics, ESD for EGC was attempted but failed because of submucosal pus, and APC was performed. During regular 6-month follow-ups, we found local recurrence at the APC site. The patient underwent subtotal gastrectomy with lymph node dissection, which was successful.
Considering the risk of incomplete treatment immediately after recovery from PG, and that recurrent disease can be more difficult to manage, as well as the characteristics of the tumor (small, well-differentiated mucosal cancer), delaying treatment and evaluation until after full recovery of PG (i.e., 3 months later) might be a better option in this particular clinical situation.
Figures and Tables
Fig. 1
Development and diagnosis of phlegmonous gastritis. Initial endoscopy shows a 1.2-cm ill-defined lesion suspicious for early gastric cancer at the distal antrum (A) and superficial gastritis at the proximal antrum (B). (C) Abdominal computed tomography shows diffuse extensive gastric wall edema and an air bubble in the antrum. (D, E) Endoscopy was repeated a day after initial endoscopy, and shows a diffusely edematous gastric wall compatible with phlegmonous gastritis and biopsy-induced mucosal erosion.
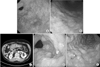
Fig. 2
Endoscopic treatment for early gastric cancer. (A) Endoscopy (9 days after admission) shows diffusely edematous mucosa and whitish purulent discharge at precutting for endoscopic submucosal dissection. (B) Argon plasma coagulation for treatment of early gastric cancer.
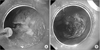
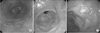
Fig. 3
Endoscopic findings after argon plasma coagulation (APC). (A) Endoscopy 3 months after APC shows resolution of edematous gastric mucosa and purulent discharge, with APC scars. (B) Endoscopy 6 months after initial APC shows recurrent adenocarcinoma at the previous APC site, and APC was repeated. (C) Endoscopy 24 months after initial APC shows recurrent early gastric cancer at the previous APC site.
References
1. Starr A, Wilson JM. Phlegmonous gastritis. Ann Surg. 1957; 145:88–93.
2. Iwakiri Y, Kabemura T, Yasuda D, Okabe H, Soejima A, Miyagahara T, et al. A case of acute phlegmonous gastritis successfully treated with antibiotics. J Clin Gastroenterol. 1999; 28:175–177.
3. Rada-Palomino A, Muñoz-Duyos A, Pérez-Romero N, Vargas-Pierola H, Puértolas-Rico N, Ruiz-Campos L, et al. Phlegmonous gastritis: a rare entity as a differential diagnostic of an acute abdomen. Description of a case and a bibliographic review. Rev Esp Enferm Dig. 2014; 106:418–424.
4. Jung SJ, Cho SJ, Choi IJ, Kook MC, Kim CG, Lee JY, et al. Argon plasma coagulation is safe and effective for treating smaller gastric lesions with low-grade dysplasia: a comparison with endoscopic submucosal dissection. Surg Endosc. 2013; 27:1211–1218.
5. Min SY, Kim YH, Park WS. Acute phlegmonous gastritis complicated by delayed perforation. World J Gastroenterol. 2014; 20:3383–3387.
6. Kim NY, Park JS, Lee KJ, Yun HK, Kim JS. A case of acute phlegmonous gastritis causing gastroparesis and cured with medical treatment alone. Korean J Gastroenterol. 2011; 57:309–314.
7. Wakayama T, Watanabe H, Ishizaki Y, Okuyama T, Ogata H, Tanigawa K, et al. A case of phlegmonous esophagitis associated with diffuse phlegmonous gastritis. Am J Gastroenterol. 1994; 89:804–806.
8. Kim GY, Ward J, Henessey B, Peji J, Godell C, Desta H, et al. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005; 61:168–174.
9. Nicholson BW, Maull KI, Scher LA. Phlegmonous gastritis: clinical presentation and surgical management. South Med J. 1980; 73:875–877.
10. Morimoto M, Tamura S, Hayakawa T, Yamanishi H, Nakamoto C, Nakamoto H, et al. Phlegmonous gastritis associated with group A streptococcal toxic shock syndrome. Intern Med. 2014; 53:2639–2642.
11. Miller AI, Smith B, Rogers AI. Phlegmonous gastritis. Gastroenterology. 1975; 68:231–238.
12. Saito M, Morioka M, Kanno H, Tanaka S. Acute phlegmonous gastritis with neutropenia. Intern Med. 2012; 51:2987–2988.
13. Corti M, Metta H, Palmieri O, Schtirbu R. Acute phlegmonous gastritis in a patient with AIDS. Enferm Infecc Microbiol Clin. 2007; 25:218–220.
14. Ajibe H, Osawa H, Yoshizawa M, Yamamoto H, Satoh K, Koinuma K, et al. Phlegmonous gastritis after endoscopic submucosal dissection for early gastric cancer. Therap Adv Gastroenterol. 2008; 1:91–95.
15. Itonaga M, Ueda K, Ichinose M. Phlegmonous gastritis caused by endoscopic ultrasound-guided fine-needle aspiration (EUSFNA). Dig Endosc. 2012; 24:488.
16. Lee BS, Kim SM, Seong JK, Kim SH, Jeong HY, Lee HY, et al. Phlegmonous gastritis after endoscopic mucosal resection. Endoscopy. 2005; 37:490–493.
17. Avilés JF, Fernández-Seara J, Bárcena R, Domíinguez F, Fernández C, Ledo L. Localized phlegmonous gastritis: endoscopic view. Endoscopy. 1988; 20:38–39.
18. Sood BP, Kalra N, Suri S. CT features of acute phlegmonous gastritis. Clin Imaging. 2000; 24:287–288.
19. Park CW, Kim A, Cha SW, Jung SH, Yang HW, Lee YJ, et al. A case of phlegmonous gastritis associated with marked gastric distension. Gut Liver. 2010; 4:415–418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download