Abstract
Purpose
Materials and Methods
Results
Conclusions
Figures and Tables
Fig. 1
Relationship between the insertion time and the postoperative duration. Error bars represent the standard deviation.

Fig. 2
(A) Relationship between the insertion time and the bowel clearance score in control group. (B) Relationship between the insertion time and the bowel clearance score in gastrectomy group. *P-value compared with ANOVA. †P-value compared with t-test.
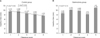
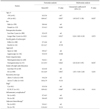
Table 1
Demographics of the control and the gastrectomy groups

Values are presented as mean±standard deviation or percent (number). DG = distal gastrectomy; PPG = pylorus preserving gastrectomy; PG = proximal gastrectomy; T-colon = transverse colon. *Classification according to the 7th edition of the American Joint Committee on Cancer. †Abscess, complicated fluid collection, and anastomosis leakage. ‡Postoperative adjuvant chemotherapy regardless of the type of drug for at least one cycle.
Table 3
Risk factors for increased insertion time (min). The insertion time of the control group was 8.7 ± 6.4 minutes.
Expected time increase from multivariate linear regression analysis. SD = standard deviation; CI = confidence interval; T-colon = transverse colon. *The reference value in the each subgroup analysis. †P<0.05. ‡Classification according to the 7th edition of the American Joint Committee on Cancer. §Abscess, complicated fluid collection, and anastomosis leakage. ∥Postoperative adjuvant chemotherapy regardless of the type of drug for at least one cycle.
Table 4
Risk factors for increased failure rate (%) of cecal intubation, as well as for very poor bowel clearance rate. The failure rate and poor bowel clearance rate of the control group were 0.07% and 7.6%.

Values are presented as percent (number). T-colon = transverse colon. *The reference value in the each subgroup analysis. †Classification according to the 7th edition of the American Joint Committee on Cancer. ‡Abscess, complicated fluid collection, and anastomosis leakage. §Postoperative adjuvant chemotherapy regardless of the type of drug for at least one cycle.
Table 5
The risk factors for "difficult/incomplete colonoscopy". The difficult/incomplete rate of the control group was 21.3%.

CI = confidence interval; T-colon = transverse colon. *The reference value in the each subgroup analysis. †P<0.05. ‡Classification according to the 7th edition of the American Joint Committee on Cancer. §Abscess, complicated fluid collection, and anastomosis leakage. ∥Postoperative adjuvant chemotherapy regardless of the type of drug for at least one cycle.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download