Abstract
Gastric hyperplastic polyps are generally considered benign lesions, although rare cases of adenocarcinoma have been reported. Although, the underlying mechanism of carcinogenesis in gastric hyperplastic polyps is still uncertain, most malignant polyps are seen to originate from dysplastic epithelium rather than from hyperplastic epithelium. Herein, we report the case of a woman diagnosed with adenocarcinoma that originated from a hyperplastic gastric polyp that was successfully removed by endoscopic submucosal dissection. In this case, we observed adenomatous changes around the cancerous component.
Hyperplastic polyps are the most common type of non-neoplastic gastric polyps.1 Although, the pathogenesis of hyperplastic polyps has not been established, it has been suggested that reparative and regenerative responses contribute significantly.2 Hyperplastic polyps of gastric origin are usually considered benign lesions and are treated as such. However, rare cases (0.6% to 2.1%) of malignant adenocarcinoma have been reported.3 Hyperplastic polyps thus have some malignant potential.
Herein, we report the case of a woman diagnosed with adenocarcinoma originating from a gastric hyperplastic polyp. Histopathological examination of the biopsy specimen revealed co-existence of hyperplastic epithelium, tubular adenoma, and adenocarcinoma.
A 74-year-old woman was referred to the gastroenterology department to undergo endoscopic treatment for gastric adenoma. Hypertension had been diagnosed 20 years ago, and the patient was being treated with antihypertensive medication. Subsequently, diabetes mellitus was newly diagnosed 3 years ago, and atrial fibrillation, 2 years ago. The patient was prescribed an oral hypoglycemic agent and aspirin. Her family history was unremarkable, and she was a housekeeper. She did not complain of any symptoms. Although, the patient looked chronically ill on physical examination, no palpable mass or tenderness was noted. Her blood pressure was 110/60 mmHg; heart rate, 72 beats/min; respiration rate, 20 breaths/min; and body temperature, 36.4℃. Complete blood count results showed that her white blood cell count was 4880/mm3; hemoglobin level, 9.9 g/dl; and platelet level, 261,000/mm3. Blood chemistry analysis showed that level of aspartate aminotransferase was 19 IU/L; alanine aminotransferase, 14 IU/L; total protein, 5.7 g/dl; albumin, 3.4 g/dl; blood urea nitrogen, 27.8 mg/dl; creatinine, 1.04 mg/dl; and fasting blood glucose, 98 mg/dl. Tumor marker levels were not evaluated. Urinalysis results were normal.
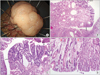
Endoscopic examination of the upper digestive tract revealed a pedunculated gastric polyp, approximately 30 mm in size, in the antrum. The polyp was red, with a distorted pit pattern at the apex (Fig. 1A, B). Because this was the first endoscopic examination that the patient had undergone, we could not compare the size or shape of the polyp. Analysis of the forcep biopsy specimen obtained from the top of this polyp indicated tubular adenoma with high-grade dysplasia, and therefore, the patient subsequently underwent endoscopic submucosal dissection (ESD) (Fig. 1C, D). The fungating lesion, 30×20 mm in size, was completely resected with a safe lateral margin (Fig. 2A).
Histological examination of the ESD specimen showed that a part of the polyp surface was replaced with moderately differentiated, intestinal-type adenocarcinoma. The cancerous lesion was limited to the mucosal layer. In the specimen, tubular adenomatous changes were also observed near the hyperplastic epithelium. In addition, adenocarcinomatous components were observed near the tubular adenoma structure (Fig. 2B~D). Neither blood vessel invasion nor lymphatic vessel invasion was noted. The course of the patient after ESD was uneventful.
Gastric hyperplastic polyps are characterized by hyperplastic, elongated, or dilated foveolar epithelium within the lamina propria.4 Hyperplastic polyps have been reported in association with various types of chronic gastritis, particularly in autoimmune gastritis,5,6 in Helicobacter pylori gastritis,7-9 and in the postantrectomy stomach.10 Although, most gastric hyperplastic polyps are benign, there have been increasing reports on adenocarcinomas arising within hyperplastic polyps.11,12 Such neoplastic transformation of gastric hyperplastic polyps is significantly associated with their size (>1 cm), their pedunculated shape, the postgastrectomy state, and the presence of a synchronous neoplastic lesion.13
The histologic criteria of Nakamura for the malignant transformation of hyperplastic polyps are as follows: 1) coexistence of benign and malignant lesions in the same polyp; 2) sufficient evidence that the malignant lesion had previously been a benign polyp; and 3) sufficient cellular and structural atypia in the malignant lesion. Polyps found to be positive for these criteria are to be diagnosed as cancerous.14
Although, the exact mechanism of carcinogenesis in gastric hyperplastic polyps is still uncertain, malignant changes in polyps are thought to arise from dysplastic epithelium rather than from hyperplastic epithelium.11,15 A few previous studies of gastric hyperplastic polyps have theorized that malignant changes develop via a hyperplasia-dysplasia-carcinoma sequence.12 In this case, both tubular adenoma and adenocarcinoma were reported in the same specimen, and therefore, the sequential process of malignant transformation in hyperplastic polyps could be directly observed.
In the present case, the polyp was removed completely by ESD and the patient did not require any additional surgery because the tumor was limited to the mucosal layer and no blood or lymphatic vessel invasion was noted. The patient has been under close observation at periodic intervals after the procedure, and no evidence of disease or recurrence has been noted at 3 months after ESD.
In conclusion, we report a gastric adenocarcinoma that arose from a hyperplastic polyp with adenomatous changes. This case and our findings support the existing hypothesis of a sequential progression from a benign polyp to cancer.
Figures and Tables
Fig. 1
Endoscopic findings. (A) Endoscopic appearance of the pedunculated gastric hyperplastic polyp (32×30 mm in size) noted at antrum. The polyp was redish and the surface was irregular. (B) The hyperplastic polyp was dyed with indigo carmine. (C) Resection was performed by the method of endoscopic submucosal dissection. (D) Endoscopic finding after en bloc resection.

Fig. 2
Pathologic findings. (A) The resected specimen obtained by endoscopic submucosal dissection. (B) Adenomatous change (between arrows) in the background of hyperplastic polyp is note (H&E, ×12.5). (C) There are foci with tubular adenoma with high-grade dysplasia (between arrows) (H&E, ×40). (D) Foci of carcinomatous transformation (black arrow) adjacent to the adenomatous lesion (red arrow) in sequence is observed (H&E, ×40).
References
1. Morais DJ, Yamanaka A, Zeitune JM, Andreollo NA. Gastric polyps: a retrospective analysis of 26,000 digestive endoscopies. Arq Gastroenterol. 2007. 44:14–17.

3. Kang HM, Oh TH, Seo JY, Joen TJ, Seo DD, Shin WC, et al. Clinical factors predicting for neoplastic transformation of gastric hyperplastic polyps. Korean J Gastroenterol. 2011. 58:184–189.

4. Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B. British Society of Gastroenterology. The management of gastric polyps. Gut. 2010. 59:1270–1276.

5. Snover DC. Benign epithelial polyps of the stomach. Pathol Annu. 1985. 20:303–329.
6. Hirasaki S, Suzuki S, Kanzaki H, Fujita K, Matsumura S, Matsumoto E. Minute signet ring cell carcinoma occurring in gastric hyperplastic polyp. World J Gastroenterol. 2007. 13:5779–5780.

7. Varis O, Laxén F, Valle J. Helicobacter pylori infection and fasting serum gastrin levels in a series of endoscopically diagnosed gastric polyps. APMIS. 1994. 102:759–764.

8. Ljubicić N, Banić M, Kujundzić M, Antić Z, Vrkljan M, Kovacević I, et al. The effect of eradicating Helicobacter pylori infection on the course of adenomatous and hyperplastic gastric polyps. Eur J Gastroenterol Hepatol. 1999. 11:727–730.

9. Ohkusa T, Takashimizu I, Fujiki K, Suzuki S, Shimoi K, Horiuchi T, et al. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, clinical trial. Ann Intern Med. 1998. 129:712–715.

10. Veereman Wauters G, Ferrell L, Ostroff JW, Heyman MB. Hyperplastic gastric polyps associated with persistent Helicobacter pylori infection and active gastritis. Am J Gastroenterol. 1990. 85:1395–1397.
11. Davaris P, Petraki K, Archimandritis A, Haritopoulos N, Papacharalampous N. Mucosal hyperplastic polyps of the stomach. Do they have any potential to malignancy? Pathol Res Pract. 1986. 181:385–389.
12. Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. Am J Gastroenterol. 1987. 82:1016–1025.
13. Nogueira AM, Carneiro F, Seruca R, Cirnes L, Veiga I, Machado JC, et al. Microsatellite instability in hyperplastic and adenomatous polyps of the stomach. Cancer. 1999. 86:1649–1656.

14. Han AR, Sung CO, Kim KM, Park CK, Min BH, Lee JH, et al. The clinicopathological features of gastric hyperplastic polyps with neoplastic transformations: a suggestion of indication for endoscopic polypectomy. Gut Liver. 2009. 3:271–275.

15. Orlowska J, Jarosz D, Pachlewski J, Butruk E. Malignant transformation of benign epithelial gastric polyps. Am J Gastroenterol. 1995. 90:2152–2159.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download