Abstract
Ewing's sarcoma is a neoplasm of the undifferenciated small round cells, which generally affects the bone and deep soft tissues of children and adolescents. We present a case of gastric Ewing's sarcoma; a 35-year-old female who had no symptoms. While she was at a routine medical checkup, a protruding mass in her gastric antrum was incidentally found on esophagogastroduodenoscopy. Endoscopic ultrasonogram showed a submucosal mass on the same lesion and a laparosopic wedge resection was done. Pathologic gross findings showed a granular grape appearance tissue and histoloigc examination revealed a small round cell tumor with CD 99 immunoexpression positive. In general, a combined modality therapy for Ewing's sarcoma such as surgical resection with chemotherapy, is accepted as an effective method. However, this patient had no adjuvant chemotherapy after surgery and she has no recurrence for eleven months.
Extraskeletal Ewing's sarcoma (ES) is a small round cell tumor that occurs in extraskelectal tissue. This has the same genetic origin as ES, which shows up as an (11;22) (q24;q12) translocation (Ewing's sarcoma gene/friend leukemia intergration 1 transcription factor gene [EWS/FLI-1] fusion; which fuses the ES gene of chromosome 22 to friend leukemia intergration 1 transcription factor gene of chromosome 11), so that it is generally categorized as an Ewing's family tumor together with ES.(1,2)
A 35-year-old female who had no special symptoms or past history of cancer was referred for surgical intervention of a protruding mass in her gastric antrum which was incidentally found by Esophagogastroduodenscopy, while she was at a routine medical checkup. Endoscopic examination showed a protruding mass of about 2.5 cm size with a central depression and an erythematous change in the anterior wall of the proximal antrum (Fig. 1A). Endoscopic ultrasonogram showed a submucosal tumor noted on the lesion. A laparoscopic gastric wedge resection was done since there was no lymph node enlargement or invasion to other organs. The pathologic gross finding showed a 5.5 cm sized granular tissue with a grape-like appearance (Fig. 1B). Histologic examination revealed undifferentiated small round cells containing round and oval nuclei (Fig. 2). Strong membranous CD 99 was noted by immunohistologic study, which is essential for diagnosis of ES. Synaptophysin was also strong by immunohistologic study but chromogranin A, cytokeratin, and desmin were negative (Fig. 3). Fluorescent in situ hybridization for detecting the t(11;22) EWSR1 gene was negative. The patient did not receive any adjuvant chemotherapy or radiotherapy and she remains symptom free and without recurrence for eleven months.
ES is a neoplasm of undifferentiated small round cells, which generally affects the bone and deep soft tissue of children and adolescents. The most commonly affected site is skeletal tissue but it has been reported that it could be present in extraskeletal tissues such as small the bowel, esophagus, vagina, pancreas or kidney. This case of ES occurs in the stomach, which is very rare. To date, the number of reported cases of ES in the stomach in the literature remains small with fewer than three being described, to the authors' knowledge. We herein report the fourth known case of gastric ES.(6-8) ES histologically is a small round cell tumor and it is CD99 positive by immunohistochemistry. It has a genetic mutation t(11;22)(q24;q12) translocation (EWS/FLI-1 fusion) that can be seen by fluorescence in situ hybridization (FISH) or reverse transcription polymerase chain reaction (RT-PCR). These traits may be essential criteria for diagnosis in most cases of ES. But there is a small chance to be negative for the t(11;22)(q2;q12) translocation by FISH or RT-PCR because both of them have chance to be negative in ES, about 3% of time in FISH and 19% in RT-PCR.(9,10)
The mainstay of ES's treatment is surgery and chemotherapy.(2) Chemotherapy has become the mainstay of ES treatment due to the high recurrence rate and difficulty in obtaining a resection negative margin when it has occurred in skeletal tissue and deep soft tissue. But based on its toxicity, compliance and effectiveness in the patient, it could be excluded if it is sure that there is no metastasis to other sites and a negative resection margin is possible. So we reviewed several articles to determine whether chemotherapy would be required or not. Most of the cases mentioned chemotherapy in addition to surgery and had better prognosis than surgery only Table 1. In this case, we only performed a wedge resection as the treatment. There were several reasons for this. First, the impression of this case was gastrointestinal stromal tumor of stomach, which was diagnosed by endoscopic ultrasonogram before the pathological diagnosis was made. Second, there was no metastasis or enlarged lymph nodes. Third, we could perform just a wedge resection to obtain free margin because it was well demarcated. Finally, the patient wanted to have a child.
To further refine our search we selected similar cases that involved the gastrointestinal tract and compared these with each other Table 1. The total number of cases was seven, which we classified into 3 different groups and are listed in Table 1. Group A was defined as those whom had chemotherapy with mention of metastasis and vascular invasion to the adjacent bowel (No 1, 2, 3).(3,4,6) Group B was those who had chemotherapy without mention of metastasis (No 4, 5).(7,8) Group C, the third and last group, was those who had no chemotherapy and no metastasis (No 6, 7).(5) In group A, despite the fact that they underwent chemotherapy additionally, there was only one survivor among three patients. This may be because they were in far advanced stage. In group B, there was no distant metastasis and invasion but they underwent an additional chemotherapy like group A. Unlike group A however, there were no deaths during the follow-up period. In group C, Patients had no metastasis and invasion, nor did the undergo chemotherapy. But there were also no deaths. Although 4 cases were in groups B and C, it suggested that there was no death after proper surgical management, regardless of an additional chemotherapy. Therefore it is necessary to consider the efficacy of the chemotherapy in groups B and C (no distant metastasis nor invasion) whether it is essential or not.
The patient who is case No. 7 did not receive any adjuvant chemotherapy or radiotherapy and remains symptom free and without recurrence for eleven months. It is required to review prospective data of these patient groups (B and C) to speak about prognosis of each group, regretfully there isn't more data regarding these groups.
In this case, our patient has regular medical check ups and computed tomography or esophago-gastro-duodenoscopy is done in every visit to evaluate for recurrence. If a recurrence in this patient is found during regular medical check up, additional chemotherapy will be mandatory for treatment.
Figures and Tables
Fig. 1
(A) Endoscopic view. A 2.5 cm sized protruding mass with central depression and erythematous change is noted in the anterior wall of proximal antrum just below angle. (B) Intraoperative laparoscopic view. Granular grape appearance mass is noted on laparoscopic view.
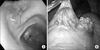
Fig. 2
Relativley monomorphic small cells with round or oval nuclei with well defined nuclear membrane (H&E, ×200).

Fig. 3
(A) Immunohistology. CD99 (+) (×400). (B) Immunohistology. Synaptophysin (+) (×400). (C) Immunohistology. Chromogranin A (-) (×400). (D) Immunohistology. Cytokeratin (-) (×400). (E) Immunohistology. Desmin (-) (×400).

Table 1
Pathologic and clinical characteristics and outcome of patients with Ewing's sarcoma

Chemo tx = chemotherapy; F/u = follow up; M = male; F = female; SM = small bowel mesenstery; SB = small bowel; SG = subtotal gastrectomy; OSBR = omentectomy and small bowel resection; SBR = small bowel resection; LGWR = laparoscopic gastric wedge resection. *Written size is the longest length of mass (cm); †this is follow-up duration after treatment at that time of publishing (month); ‡vascular invasion was noted on histologic examination; §adjusant small bowel serosa invaision was noted on histologic examination; ∥there was not any mention of metastasis or invasion.
References
1. El Weshi A, Allam A, Ajarim D, Al Dayel F, Pant R, Bazarbashi S, et al. Extraskeletal Ewing's sarcoma family of tumours in adults: analysis of 57 patients from a single institution. Clin Oncol (R Coll Radiol). 2010. 22:374–381.

3. Soulard R, Claude V, Camparo P, Dufau JP, Saint-Blancard P, Gros P. Primitive neuroectodermal tumor of the stomach. Arch Pathol Lab Med. 2005. 129:107–110.

4. Czekalla R, Fuchs M, Stölzle A, Nerlich A, Poremba C, Schaefer KL, et al. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol. 2004. 16:1391–1400.

5. Colovic RB, Grubor NM, Micev MT, Matic SV, Atkinson HD, Latincic SM. Perigastric extraskeletal Ewing's sarcoma: a case report. World J Gastroenterol. 2009. 15:245–247.

6. Shek TW, Chan GC, Khong PL, Chung LP, Cheung AN. Ewing sarcoma of the small intestine. J Pediatr Hematol Oncol. 2001. 23:530–532.

7. Adair A, Harris SA, Coppen MJ, Hurley PR. Extraskeletal Ewings sarcoma of the small bowel: case report and literature review. J R Coll Surg Edinb. 2001. 46:372–374.
8. Kie JH, Lee MK, Kim CJ, Lee K, Kwon KW, Yang WI. Primary Ewing's sarcoma of the suodenum: a case report. Int J Surg Pathol. 2003. 11:331–337.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download