Abstract
Purpose
Although serosal invasion is a critical predisposing factor for peritoneal dissemination in advanced gastric cancer, the accuracy of preoperative assessment using routine imaging studies is unsatisfactory. This study was conducted to identify high-risk group for serosal invasion using preoperative factors in patients with advanced gastric cancer.
Materials and Methods
We retrospectively analyzed clinicopathological features of 3,529 advanced gastric cancer patients with Borrmann type I/II/III who underwent gastrectomy at Korea Cancer Center Hospital between 1991 and 2005. We stratified patients into low- (≤40%), intermediate- (40~70%), and high-risk (>70%) groups, according to the probability of serosal invasion.
Results
Borrmann type, size, longitudinal and circumferential location, and histology of tumors were independent risk factors for serosal invasion. Most tumors of whole stomach location or encircling type had serosal invasion, so they belonged to high-risk group. Patients were subdivided into 12 subgroups in combination of Borrmann type, size, and histology. A subgroup with Borrmann type II, large size (≥7 cm), and undifferentiated histology and 2 subgroups with Borrmann type III, large size, and regardless of histology belonged to high-risk group and corresponded to 25% of eligible patients.
Conclusions
This study have documented high-risk group for serosal invasion using preoperative predictors. And risk stratification for serosal invasion through the combination with imaging studies may collaboratively improve the accuracy of preoperative assessment, reduce the number of eligible patients for further staging laparoscopy, and optimize therapeutic strategy for each individual patient prior to surgery.
Peritoneal metastasis, from gastric cancer, is the most typical non-curative factor in patients with advanced gastric cancer (AGC). It commonly occurs when tumor cells are released into the abdominal cavity after the tumor is exposed, following serosal invasion.(1-3) Except for the cases with severe peritoneal metastasis, such as carcinomatosis, as it is very difficult to diagnose peritoneal metastasis from gastric cancer via a preoperative examination on disease stage, peritoneal metastasis is often found during surgery for a radical gastrectomy. Thus, some researchers suggested that diagnostic laparoscopy should be conducted on all patients with AGC.(4,5) However, an incidence rate of peritoneal metastasis is 4~23% in patients with AGC, which is relatively low; the conduct of diagnostic laparoscopy in all the patients wastes time and money(6-8) Thus, it is most desirable that patients with high risk of peritoneal metastasis should be selected before surgery among patients with AGC, and then diagnostic laparoscopy should be conducted on the selected patients.
In peritoneal metastasis from AGC, its incidence rate increases as T and N stage increases. In particular, serosal invasion by tumors is the most critical factor.(9,10) Thus, patients with AGC, which shows serosal invasion in preoperative examinations for clinical stage, are considered subjects who are suitable for diagnostic laparoscopy. However, computed tomography (CT) and gastric endoscopic ultrasound, which are mainly used for diagnosing the invasion depth of gastric cancer before surgery, showed that their sensitivity to serosal invasion was 40% and 55%, respectively.(11,12)
Accordingly, this study was retrospectively conducted on patients who had undergone radical gastrectomy, due to Borrmann I/II/III type AGC, to analyze the correlation of preoperatively clininopathological parameters and serosal invasion, and to stratify the serosal invasion risk of AGC by combining the statistically significant parameters.
This study was retrospectively conducted on 3,529 patients (2,395 males, 1,134 female, male to female ratio 2.1 : 1) with Borrmann I/II/III type gastric cancer, among the patients who had undergone radical gastrectomy at the Korea Cancer Center Hospital from 1991 to 2005, and who had been diagnosed with AGC (>T1) in a histological examination. Patients that were excluded were those with Borrmann IV type, who had a high risk of serosal invasion, and those with Borrmann V type, who has a low risk of serosal invasion.(13-16)
The patient's gender, age, Borrmann type, tumor size, longitudinal and horizontal location of tumor, histologic grade, and tumor invasion depth were retrospectively reviewed, using pathological reports and medical records. The longitudinal location of tumor was classified into the upper 1/3, middle 1/3, lower 1/3, and whole type. The horizontal location of tumor was classified into lesser curvature, anterior, greater curvature, posterior, and encircling type. Histologic type of tumor was classified into the differentiated type, which includes papillary adenocarcinoma, well differentiated tubular adenocarcinoma, and moderately differentiated tubular adenocarcinoma, as well as the undifferentiated type, which includes poorly differentiated tubular adenocarcinoma, signet ring cell adenocarcinoma, mucinous adenocarcinoma, and other adenocarcinomas.
The subjects were classified into the negative group in which the tumor invasion was limited to the muscularis propria or subserosa (1,208 patients), and the positive group with serosal invasion (2,321 patients), according to the presence of serosal invasion (visceral peritoneum or adjacent structure), and then the correlation of clinicopathological parameters that can be assessed before surgery with serosal invasion was analyzed. In addition, after the subjects were classified via the combination of the statistically significant risk factors of serosal invasion, the positive rate of serosal invasion was examined for each group. The subjects were classified into the low-risk, intermediate-risk, and high-risk groups if the positive rate of serosal invasion ≤40%, 40~70%, and >70%, respectively, followed by stratification, according to serosal invasion risk. In addition, the overall survival curve was compared, among the 3 groups. For the follow-up of the patients, interview, physical examination, tumor markers, plain chest X-ray and abdominal CT were conducted every 3~4 months, for 2 years after the surgery; and thereafter, they were conducted every 6 months. Gastric endoscopy or upper gastrointestinal series was conducted at least once a year. Follow-up period, death, and reasons for death were examined using the patient's medical records and statistics, such as the Korea cancer registry program.
A statistical analysis was conducted, using SPSS for Window version 13.0 (SPSS Inc., Chicago, IL, USA). A Chi-square test was used for univariate analysis of nominal variables, and logistic regression was used for multivariate analysis. A survival analysis was conducted, using the Kaplan-Meier survival curve and Log-rank test. If P<0.05, it was considered statistically significant.
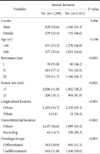
Univariate and muitivariate analysis showed that among the clinicopathological parameters, the Borrmann type, tumor size, tumor's longitudinal and horizontal location, and histologic grade had a statistically significant correlation with serosal invasion by tumor; however, the patient's gender and age had no correlation with serosal invasion (Table 1, 2).
Of the total 3,529 patients, the serosal invasion rate was shown to be 46.2% in patients with Borrmann I type (n=130, 3.7%), 62.9% in patients with Borrmann II type (n=1,114. 31.6%), and 68.3% in patients with Borrmann III type (n=2,285, 64.7%), which showed that the serosal invasion rate was high in patients with Borrmann II and III. The odd ratio of serosal invasion was shown to be 2.3-fold and 3.1-fold higher in patients with Borrmann II and III, respectively, than in the patients with Borrmann I type. In addition, when patients were divided into the 2 groups, according to the reference tumor size of 7 cm, and the serosal invasion rate was then compared between the 2 groups, the serosal invasion rate was shown to be 58.2% in the group with tumor size <7 cm and 81.9% in the group with tumor size ≥7 cm. Furthermore, the odd ratio of serosal invasion was also shown to be 3.1-fold higher in the group with tumor size ≥7 cm than in the group with tumor size <7 cm.
When the serosal invasion rate was compared, according to the longitudinal location of the tumor, no significant difference was found, according to the location of the upper, middle, and lower location. However, most of the patients with the whole type, which invades the 3 areas from the upper to the lower stomach, and comprises only 1.5% (54 cases) of the total patients, showed serosal invasion positivity. Meanwhile, when the serosal invasion rate was compared according to the horizontal location of the tumor, no significant difference was found, according to the location of lesser curvature, anterior, greater curvature, and posterior. However, it was shown to be 85.3% in the patients with encircling type, which was significantly higher than that of other tumors, and the odd ratio was also shown to be 2.5-fold higher in patients with encircling type than in patients with other types. When the serosal invasion rate was compared according to histologic classification of tumor, it was shown to be 61.1% in the patients with differentiated type and 69.9% in the patients with undifferentiated type, which showed a statistically significant difference, despite an odd ratio of 1.2-folds.
After the subjects were classified via the combination of the statistically significant risk factors of serosal invasion, the positive rate of serosal invasion was examined in each group. However, as the serosal invasion rate was 94.4% and 85.3% in patients with whole type and patients with encircling type, respectively, which were very high, they were assigned to the high risk group by a single factor. The subjects were classified according to the combination of three factors: Borrmann type, tumor size, and histologic grade, and then the serosal invasion rate of each group were examined (Table 3).
Each group was classified into the following; low-risk, intermediate-risk, and high-risk groups according to the positive rate of serosal invasion ≤40%, 40~70%, and >70%, respectively (Fig. 1). The serosal invasion rate was shown to be 21~32% in the group with tumor size <7 cm and Borrmann I type, corresponding to the low risk group. Meanwhile, it was shown to be 84~87% in the group with tumor size ≥7 cm and Borrmann III type and group with tumor size ≥7 cm, Borrmann II type, and undifferentiated type, which corresponded to the high risk group. The classification of serosal invasion risk showed that 25% of the total patients corresponded to the high risk group. In addition, when the overall survival curve was compared via the comparison of prognosis among the three groups, a poor prognosis was shown in the high risk, intermediate risk, and low risk groups, in that order (Fig. 2).
Peritoneal metastasis has been known to be the most common non-curative factor in patients with AGC. An incidence rate of peritoneal metastasis has been reported to be approximately 4~23% in open surgery, which is assessed to be resectable in a preoperative assessment.(6-8) In this case, problems such as patient's suffering from surgery, possibility of complication, delayed chemotherapy, and unexpected metastasis to the wound site may occur. Due to the recent development of gastric endoscopic ultrasound and imaging studies, the accuracy of clinical staging and the diagnostic rate of metastatic lesion have been increasing. However, the diagnostic rate of peritoneal metastasis from gastric cancer is still low. Noninvasive examinations, which are mainly used for determining the clinical staging of gastric cancer, include abdominal CT, positron emission tomography-CT and gastric endoscopic ultrasound, but they have low sensitivity to peritoneal metastasis.(11,12)
Owing to the recent development of laparoscopic surgery, diagnostic laparoscopy for determining the clinical staging of AGC has been gradually increasing. Relative to a permanent histological examination on peritoneal metastasis, suspected by laparoscopic examination, the diagnostic sensitivity and accuracy of laparoscopic examination on peritoneal metastasis were reported to be 65~95% and 85~100%, respectively. The change rate of the treatment of patients with AGC by diagnostic laparoscopy, and the rate of preventing unnecessary open surgery have been reported to be 10~60% and 10~45%, respectively.(17-22) The NCCN and ESMO guidelines mentioned that all patients with gastric cancer that is expected to be resectable are subjects for the indication of diagnostic laparoscopy.(19,20) Meanwhile, the SAGES guideline limited subjects for diagnostic laparoscopy to patients with suspected serosal invasion.(21) In this study, considering that an incidence rate of peritoneal metastasis is 4~23% during open surgery, the conduct of diagnostic laparoscopy on all the patients with AGC was considered inefficient, as it increases temporal and economic costs. Thus, authors thought that the SAGES guideline is more appropriate for the application of diagnostic laparoscopy in patients with AGC.
In general, peritoneal metastasis occurs by the direct invasion of tumor into the gastric wall and penetrating the serosa. Sadeghi et al.(23) reported that serosal invasion was observed in 95% of patients with peritoneal metastasis, and that the invasion was limited to the muscularis propria or subserosa, in only 5% of patients with peritoneal metastasis. Thus, if tumor invasion depth is accurately determined before surgery, the risk of peritoneal metastasis can be preoperatively assessed and an appropriate examination for determining clinical stage can be additionally conducted.
Although tumor invasion depth can be diagnosed by noninvasive imaging studies, such as abdominal CT, positron emission tomography-CT, and gastric endoscopic ultrasound, they have a sensitivity of 40%, 65%, and 55%, respectively, for the diagnosis of serosal invasion, which shows low accuracy.(10-12) Owing to the recent development of radiological devices, Spiral CT and MDCT (multidetector-row CT) with improved diagnostic accuracy have been developed, which increase diagnostic accuracy, but still have a lower accuracy for the invasion depth compared to gastric endoscopic ultrasound.(24-26)
In this study, a new approach that diagnoses serosal invasion in patients with AGC was explored. The results of this study showed that a significant correlation was found between clinicopathological parameters that can be assessed before the surgery and serosal invasion, and that the serosal invasion risk was stratified into the low-, intermediate-, and high-risk groups, via the combination of the predictors of serosal invasion. However, the prediction of serosal invasion, using the criteria of the high risk group, had 32.6% sensitivity and 89.7% specificity, which were not high enough. Thus, this method was shown to be inappropriate for diagnosing serosal invasion.
On the contrary, if diagnostic laparoscopy is selectively applied to the serosal invasion, high-risk group among patients with AGC, when the aforementioned stratification is applied to clinical practices, the frequency of conducting diagnostic laparoscopy is expected to decrease by 25%. If the conventional radiologic evaluations with this stratification are used for more accurately diagnosing the invasion depth of gastric cancer before surgery, and diagnostic laparoscopy is selectively conducted on patients with a high risk of serosal invasion, the incidence rate of peritoneal metastasis, during radical gastrectomy is expected to decrease.
In addition, the gross type of tumor, that is, the growth pattern of the tumor was shown to be correlated with serosal invasion risk, and the risk of serosal invasion increased in proportion to tumor size. Furthermore, in the cases of tumor extending to the whole stomach longitudinally or encircle the stomach circumferentially, rather than tumor location itself, concurrent serosal invasion was shown in most cases. Thus, the characteristics of tumor that can be grossly assessed, such as AGC's gross type, size, and location, were shown to be closely correlated with serosal invasion by the tumor. These gross characteristics can be easily found via endoscopy or upper gastrointestinal endoscopy before surgery.
An overall survival analysis, according to serosal invasion risk, showed that the patent's prognoses were poorer in the high-risk group than in the other groups. This result indicates that risk stratification for serosal invasion is correlated with the patient's disease stage. Based on the results of this study, an algorithm for the diagnosis and treatment of patients with AGC was presented, using the preoperative predictors (Fig. 3). In particular, diagnostic laparoscopy, including peritoneal lavage, is recommended to be conducted on the high-risk group for the accurate determination of disease stage.
The results of this study showed that the clininopathological parameters, which can be assessed before surgery, were correlated with serosal invasion in Borrmann I/II/III type patients with AGC, and that serosal invasion risk was stratified via the combination of predictors, such as Borrmann type, tumor size, and histologic grade. If the conventional imaging studies are used with this risk stratification of serosal invasion, suggested in this study, it could decrease the frequency of conducting diagnostic laparoscopy, and help build a treatment plan that is appropriate for the individual patients.
Figures and Tables
Fig. 2
Overall survival curves for low-, intermediate- and high-risk groups of serosal invasion in patients with advanced gastric cancer.

Acknowledgments
This study was supported by a grant from Ministry of Education, Science and Technology (MEST), Republic of Korea through the Program of Research and Development of Radiopharmaceuticals (50556-2011, 50556-2012).
References
1. Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003. 21:233–248.

2. Marutsuka T, Shimada S, Shiomori K, Hayashi N, Yagi Y, Yamane T, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003. 9:678–685.
3. Yonemura Y, Kawamura T, Bandou E, Tsukiyama G, Endou Y, Miura M. The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res. 2007. 169:11–23.

4. Kapiev A, Rabin I, Lavy R, Chikman B, Shapira Z, Kais H, et al. The role of diagnostic laparoscopy in the management of patients with gastric cancer. Isr Med Assoc J. 2010. 12:726–728.
5. Lehnert T, Rudek B, Kienle P, Buhl K, Herfarth C. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg. 2002. 89:471–475.

6. Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999. 178:256–262.

7. Suzuki T, Ochiai T, Hayashi H, Hori S, Shimada H, Isono K. Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg Oncol. 1999. 17:103–107.

8. Yamamoto M, Matsuyama A, Kameyama T, Okamoto M, Okazaki J, Utsunomiya T, et al. Prognostic re-evaluation of peritoneal lavage cytology in Japanese patients with gastric carcinoma. Hepatogastroenterology. 2009. 56:261–265.
9. Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000. 58:96–107.

10. Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990. 77:436–439.

11. Furukawa K, Miyahara R, Itoh A, Ohmiya N, Hirooka Y, Mori K, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011. 197:867–875.

12. Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006. 9:192–196.

13. Yook JH, Oh ST, Kim BS. Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer Res Treat. 2005. 37:87–91.

14. Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, et al. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology. 2009. 77:197–204.

15. Maehara Y, Anai H, Moriguchi S, Watanabe A, Tsujitani S, Sugimachi K. Gastric carcinoma invading muscularis propria and macroscopic appearance. Eur J Surg Oncol. 1992. 18:131–134.
16. Ichiyoshi Y, Tomoda M, Tomisaki S, Oda S, Ohno S, Maehara Y, et al. Macroscopic appearance and biological character of gastric cancer invading the muscularis propria. Hepatogastroenterology. 1996. 43:553–559.
17. Arnold JC, Neubauer HJ, Zöpf T, Schneider A, Benz C, Adamek HE, et al. Improved tumor staging by diagnostic laparoscopy. Z Gastroenterol. 1999. 37:483–488.
18. Blackshaw GR, Barry JD, Edwards P, Allison MC, Thomas GV, Lewis WG. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer. 2003. 6:225–229.

19. Roviaro GC, Varoli F, Sonnino D, Nucca O, Rabughino G, Scarduelli A. Can routine laparoscopy help to reduce the rate of explorative laparotomies for gastric cancer? Laparoscopy in gastric cancer. Diagn Ther Endosc. 2000. 6:125–131.

20. Sotiropoulos GC, Kaiser GM, Lang H, Treckmann J, Brokalaki EI, Pottgen C, et al. Staging laparoscopy in gastric cancer. Eur J Med Res. 2005. 10:88–91.
21. Smith A, Finch MD, John TG, Garden OJ, Brown SP. Role of laparoscopic ultrasonography in the management of patients with oesophagogastric cancer. Br J Surg. 1999. 86:1083–1087.

22. Yano M, Tsujinaka T, Shiozaki H, Inoue M, Sekimoto M, Doki Y, et al. Appraisal of treatment strategy by staging laparoscopy for locally advanced gastric cancer. World J Surg. 2000. 24:1130–1135.

23. Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000. 88:358–363.

24. Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010. 25:512–518.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download