Abstract
A 67 year old male at a regular checkup underwent esophagogastroduodenoscopy. On performing esophagogastroduodenoscopy, a lesion about 1.2 cm depressed was noted at the gastric angle. The pathology of the biopsy specimen revealed a well-differentiated adenocarcinoma. On performing an abdominal computed tomography (CT) scan & positron emission tomography-computed tomography (PET-CT) scan, no definite evidence of gastric wall thickening or mass lesion was found. However, lymph node enlargement was found in the left gastric and prepancreatic spaces. This patient underwent laparoscopic assisted distal gastrectomy and D2 lymph node dissection. On final examination, it was found out that the tumor had invaded the mucosal layer. The lymph node was a metastasized large cell neuroendocrine carcinoma with an unknown primary site. The patient refused chemotherapy. He opted to undergo a close follow-up. At the postoperative month 27, he had a focal hypermetabolic lesion in the left lobe of the liver that suggested metastasis on PET-CT scan. He refused to undergo an operation. He underwent a radiofrequency ablation.
Cancer of unknown primary site (CUP) represent a heterogeneous group of metastatic tumors for which a standardized diagnostic work-up fails to identify the site of origin at the time of diagnosis and account for 3~5% of all malignancies.(1) Poorly differentiated large cell neuroendocrine carcinoma (LCNEC) comprise a rare and still seldom reported subset of neuroendocrine tumors. Here, we present a rare case of LCNEC of unknown primary site, which was detected originally in a lymph node of early gastric cancer.
A 67 year old male without any significant past medical history underwent esophagogastroduodenoscopy during a routine check-up. He had no other symptoms. Physical examination was unremarkable and laboratory findings showed no abnormalities. On esophagogastroduodenoscopy, mild atrophic change with intestinal metaplasia was noted at the antrum and body. About 1.2 cm depressed lesion at the angle and about 1.5 cm mildly elevated lesion at the posterior wall of the antrum were noted. The pathology of the biopsy specimen revealed a well differentiated adenocarcinoma. On abdominal computed tomography (CT) scan, there was no definite evidence of gastric wall thickening or mass lesion. However, a lymph node enlargement was found in the left gastric and prepancreatic spaces. On positron emission tomography-computed tomography (PET-CT) scan, there was no visible hypermetabolic activity of proven gastric malignancy. Hypermetabolic activity, however, was depicted in the prepancreatic area and the left gastric area that implied metastatic lymphadenopathy (Fig. 1). The gastric tumor had invaded the mucosal layer on the endoscopic ultrasonography. This patient underwent laparoscopic assisted distal gastrectomy and D2 lymph node dissection with Billroth-I reconstruction. On the final pathologic examination, there were two early gastric cancers (Fig. 2). One tumor was 1.5×1.0 cm, an early gastric cancer (EGC) type III lesion at the lower body of the stomach. The other was a 1.2×1.0 cm EGC type IIc lesion at the angle of the stomach. The tumors were well-differentiated carcinomas that invaded the mucosal layer (T1a). Lymph node sections showed that the tumor tissue consisted of neoplastic pleomorphic large cells with a palisading, trabecula and sinusoid pattern (Fig. 3). There were frequent mitoses (more than 20/10 High Power Fields) and some necrosis. These cells were positive for CD56, chromogranin and synaptophysin on immunohistochemical stains (Fig. 4). These findings were compatible with large cell neuroendocrine carcinomas. The patient recovered well and was discharged on the postoperative day 8. The patient refused adjuvant chemotherapy and opted to undergo close follow-up. At postoperative month 27, he had a focal hypermetabolic lesion in the left lobe of the liver that suggested metastasis on PET-CT (Fig. 5). The tumor was 2.3 cm and oval shaped on magnetic resonance imaging of the liver and we therefore planned surgery. However, the patient did not want surgery, so he had radiofrequency ablation. The tumor cells were positive for cytokeratin (CK), CK7, CD56, and synaptophysin, and negative for CK20, chromogranin, and HepPar-1 on liver biopsy on immunohistochemical stains. There was no procedure-associated morbidity. He did not want platinum-based chemotherapy that required hospitalization, so he is treated with oral fluorouracil based chemotherapy.
Patients with metastatic CUP present with metastatic disease without an established primary site. The majority of patients (80%) have systemic metastases of adenocarcinomas or poorly differentiated carcinomas and appear in the poor risk CUP group. Only 20% of patients belong to favorable prognosis groups.(2)
Neuroendocrine CUP account for uncommon, diverse tumors with variable clinical behavior, predicted by tumor grade or differentiation. These carcinomas probably arise from an occult/clinically undetectable primary site in one of several locations such as bronchus, pancreas, stomach, colon, rectum and several other sites.(3)
There are two different clinicopathologic subsets of neuroendocrine tumor (NET).
The first subset includes low-grade or well differentiated tumors that are frequently indolent and should be managed similar to advanced carcinoid tumors.
The second subset includes high-grade or poorly differentiated carcinomas that are rapidly growing and aggressive but responsive to platinum based combination chemotherapy. These subsets also include small cell and large cell NETs. Poorly differentiated large cell NETs are usually not identified by routine hematoxylin and eosin light microscopy but require immunohistochemical stains (i.e. chromogranin, synaptophysin, etc.) or electron microscopy for their diagnosis.(3)
Staining for keratins CK7 and CK20 may suggest indications of a possible primary site, and staining for chromogranin A and synaptophysin is needed to profile neuroendocrine differentiation. In our case, the lymph nodes were strongly positive for chromogranin, synaptophysin, and CD56, and focal positive for CK, but the tumor cells of the metastatic liver were positive for CK, CK7, CD56, and synaptophysin, and negative for CK20 and chromogranin. The CK7+/CK20- phenotype favors the lung, breast or ovarian primary carcinoma but the number of false-positives and false-negatives makes a definitive diagnosis of a primary site difficult on this basis alone.(4-6) In our case, there was a 3~4 mm small nodule on the right upper lobe of the lung that suggested granuloma on preoperative chest CT scan and the patient underwent close follow-up for more than 2 years. However, we could not find any suspicious lesion except a focal hypermetabolic lesion in the left lobe of the liver on abdominal CT scan & PET-CT scan.
Hepatic metastases are the most powerful prognosticator of survival in patients with NET regardless of primary site.(7) Besides regional lymph nodes, the liver is the predominant site of NET metastases. Up to 75% of patients with small bowel NET and 30-85% of those with tumors localized within the pancreas present with liver metastases either at initial evaluation or during the course of their disease.(8-10) An additional 5~10% of NET patients present with liver metastases with unknown primary tumor site. A 13~54% 5 year survival in histological cohorts of patients with untreated neuroendocrine liver metastases compared with 75~99% in those free of hepatic deposits underlines the unique molecular genetics of malignant NET and clearly delineates them from their non-endocrine counterparts.(11-14)
The treatment of a NET with liver metastases includes liver resection, liver transplantation, radiofrequency ablation, hepatic transcatheter arterial embolization, peptide receptor radionuclide therapy, chemotherapy, etc. In our case, the tumor was a single lesion that was located in the lateral segment of the liver, and we planned surgery. For patients with NET liver metastases and unknown primary tumor, surgical exploration effectively identifies and resects occult primary tumors that are often located in the small intestine. Within the gastrointestinal NET, the small intestine is the most common site. Computed tomography may be useful in detecting mesenteric masses that may result from extension of the primary NET or lymph node metastases with associated fibrosis, suggesting a small-intestine primary tumor.(15) If the patient had undergone surgical exploration, we might have a chance to find a lesion that had not shown on abdominal CT scan. However, the patient did not want surgery, so he had radiofrequency ablation. There was no procedure-associated morbidity. He did not want platinum-based chemotherapy that required hospitalization, so he is being treated with oral fluorouracil based chemotherapy.
Figures and Tables
Fig. 1
Preoperative abdominal computerd tomography scan (A) and positron emission tomography-computerd tomography (B).
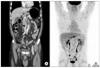
Fig. 2
The resected specimen showed two early gastric cancers. One tumor was 1.5×1.0 cm, early gastric cancer (EGC) type III lesion at the lower body of the stomach. The other was a 1.2×1.0 cm, EGC type IIc lesion at the angle of the stomach.

Fig. 3
Lymph node sections showed that the tumor tissue consisted of neoplastic pleomorphic large cells with palisading, trabecula and sinusoid pattern (hematoxylin-eosin stain, original magnification, ×12.5).

Fig. 4
The tumor cells of neuroendocrine carcinoma showed positive stains for CD56, chromogranin and synaptophysin on immunohistochemical stains (CD56, original magnification, ×100).
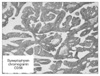
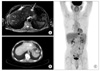
Fig. 5
Postoperative liver magnetic resonance imaging (MRI) & positron emission tomography-computerd tomography (PET-CT) scan. At postoperative month 27, the patient had a focal hypermetabolic lesion in the left lobe of the liver that suggested metastasis on PET-CT (B, C). The tumor was 2.3 cm and oval shaped on MRI over the liver (A).
References
1. Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G. ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011. 22:Suppl 6. vi64–vi68.

2. Stoyianni A, Pentheroudakis G, Pavlidis N. Neuroendocrine carcinoma of unknown primary: a systematic review of the literature and a comparative study with other neuroendocrine tumors. Cancer Treat Rev. 2011. 37:358–365.

3. Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol. 2009. 36:52–59.

4. Tot T. Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999. 85:171–177.
5. Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002. 38:758–763.
6. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003. 39:1990–2005.

7. Rindi G, D'Adda T, Froio E, Fellegara G, Bordi C. Prognostic factors in gastrointestinal endocrine tumors. Endocr Pathol. 2007. 18:145–149.

8. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003. 97:934–959.

9. Norheim I, Oberg K, Theodorsson-Norheim E, Lindgren PG, Lundqvist G, Magnusson A, et al. Malignant carcinoid tumors. An analysis of 103 patients with regard to tumor localization, hormone production, and survival. Ann Surg. 1987. 206:115–125.

10. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005. 19:753–781.

11. Starker LF, Carling T. Molecular genetics of gastroenteropancreatic neuroendocrine tumors. Curr Opin Oncol. 2009. 21:29–33.

12. McDermott EW, Guduric B, Brennan MF. Prognostic variables in patients with gastrointestinal carcinoid tumours. Br J Surg. 1994. 81:1007–1009.

13. Thompson GB, van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988. 104:1011–1017.
14. Zeitels J, Naunheim K, Kaplan EL, Straus F 2nd. Carcinoid tumors: a 37-year experience. Arch Surg. 1982. 117:732–737.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download