Abstract
Although the biological potential of gastric epithelial dysplasia (GED) as a precursor of gastric cancer has never been in doubt, the classification of these lesions has been controversial and fraught with marked variations in approach to diagnosis across the world. The complexity of cyto-architectural features has been considered to be of paramount importance for the diagnosis of carcinoma in Japan, while breach of the basement membrane and invasion into the lamina propria has been considered the sine qua non of malignancy and hence a pre-requisite for the diagnosis of cancer in the West. In Korea, although the incidence of gastric cancer is similar to Japan, the diagnostic approach to GED or cancer seems to lie midway between Western and Japanese criteria. In this review, we will discuss the difference in the diagnosis of GED and cancer between two pathologists working in the comprehensive cancer center located in Japan and Korea, one of the most prevalent areas in the world for gastric cancer.
The advent of the flexible endoscope and its world-wide use in clinical practice has had a major impact on the management of gastric cancer.(1) Histopathologic diagnosis remains the foundation of clinical decision making in the treatment of gastric neoplasia. However, based on subjective morphologic criteria, clinicians and pathologists continue to have concerns about the ability of pathologists to achieve consistent and accurate diagnoses using published criteria.(2) In 2000, a group of gastrointestinal pathologists convened in Vienna, Austria, for the purpose of developing a new system for the classification of dysplasia that would help to minimize the widely recognized discrepancies in morphological interpretation of gastric epithelial dysplasia (GED) and to reach consensus on the nomenclature.(3) However, modification of the nomenclature has not resolved the high level of intra- and interobserver variability with regard to the pathological classification of neoplasia and its mimickers. These interobserver variations are not a problem confined to Western and Japanese regions, and poor interobserver agreement in the distinction between high-grade dysplasia and adenocarcinoma in the pretreatment biopsies of Brrett's esophagus has been reported among pathologists practicing in the same institute located in United States, where Barrett's esophagus is one of the most common medical conditions.(4)
In Japan, gastric carcinoma is diagnosed on nuclear and structural criteria, even when invasion is absent according to the Western viewpoint.(5) This may also contribute to the relatively high incidence and good prognosis of gastric carcinoma in Japan compared to Western countries. In Korea, the terminology, definitions, and diagnostic criteria for GED are very heterogeneous.(6) As one of the pathologists working in a large volume hospital and handling a large number of gastric biopsy specimens, our experiences in the pathologic diagnosis of GED and carcinoma might help identify the differences between Korea and Japan.
In July 2008, to observe interobserver variation between two pathologists working in two different countries, KMK visited RK in Japan with her collection of gastric biopsy specimens with follow up information and that were associated with diagnostic difficulty. Without any knowledge of follow up or diagnosis by KMK, RK diagnosed the H&E slides of KMK's gastric biopsy specimens.
The overall differences in the diagnosis of gastric biopsies are depicted in Table 1.
In the diagnosis of regenerative atypia, although we reached agreement in most cases (Fig. 1), we disagreed on two cases. One case (Fig. 2) diagnosed as regenerative atypia by KMK was diagnosed as suspicious carcinoma by RK and the other one diagnosed as favor reactive atypia was diagnosed as atypical glands with high-grade dysplasia by KMK (Fig. 3).
In cases that are difficult to diagnose, whether they are neoplasia, dysplasia or regenerative atypia, the Japanese guideline recommends making a temporary diagnosis of 'Group 2, indefinite for neoplasia'. This corresponds to 'Category 2' of the Vienna classification (Table 2).(7,8) In case of a 'Group 2' diagnosis, pathologists should comment on the reason for the diagnosis of 'indefinite for neoplasia', and, if possible, make 'deeper sections' or perform immunohistochemical stains for p53 and MIB-1. In those cases, Japanese pathologists sometimes use the term 'borderline lesion' or suspected adenocarcinoma for a biopsy case showing dysplastic lesions histologically beyond a typical tubular adenoma with low-grade dysplasia, and recommend endoscopic therapy. Even in a case of low-grade dysplasia, if the lesion shows predominantly gastric-foveolar type differentiation or villous/papillary structures, they prefer to make a diagnosis of 'suspicious of adenocarcinoma' rather than low-grade dysplasia. Such a lesion may invade into the submucosal layer, keeping its structure, without an invasive stromal reaction within the lamina propria.
In the diagnosis of GED, we used "adenoma" in daily practice. There was general agreement on the diagnosis of adenoma with low-grade dysplasia. Glands in adenomas resembled colonic adenomas with crowded, tubular glands lined by atypical columnar cells with pencillate, hyperchromatic nuclei, with pseudostratification and inconspicuous nucleoli, mucin depletion, and lack of surface maturation.(3) Tumor glands show minimal architectural disarray and only mild to moderate cytological atypia and nuclei are located in the basal part of the glands (Fig. 4). However, several cases diagnosed as adenoma with low-grade dysplasia by KMK were diagnosed as adenoma with high-grade dysplasia by RK. For the diagnosis of adenoma with low-grade dysplasia, RK suggested that glands should be straight without branching, torsion and budding, and the nuclei should show spindling. However, KMK diagnosed adenoma with low-grade dysplasia based on criteria proposed by a study group of Korean gastrointestinal pathologists irrespective of glandular structural anomalies; the length of the nuclei should be lower than half of the height of the tumor cells and at least three contiguous glands should show these cytologic abnormalities.(9) In four cases diagnosed as adenoma with high-grade dysplasia, RK used the term "very well differentiated intramucosal intestinal type adenocarcinoma without invasion" (Fig. 5).
In cases diagnosed as invasive adenocarcinoma, distinct structural anomalies, such as marked glandular crowding, excessive branching, and budding were evident. Intraluminal necrotic debris was also common. Single tumor cells or clusters of them infiltrated within the lamina propria in the absence of desmoplasia. The neoplastic cells in intramucosal invasive neoplasia are usually cuboidal with a high nucleus to cytoplasm ratio. Round nuclei with prominent nucleoli and marked loss of polarity are common.(3) Mitoses are usually numerous and atypical mitoses can be identified. RK diagnosed adenocarcinoma in the absence of invasion into the lamina propria and thought that round oval nuclei found at the bottom or surface of foveolar epithelium with prominent nucleoli are adequate for the diagnosis of carcinoma. However, in cases with no definite invasion, KMK diagnosed them as adenoma with high-grade dysplasia (Fig. 6). This trend was more evident in histology when the tumor was gastric foveolar phenotype (Fig. 7). In Japan, the differential diagnosis between adenoma and adenocarcinoma is made on the basis of cellular and structural atypia. Even for small biopsy specimens, Japanese pathologists diagnose carcinoma if the tumor shows the same cellular and/or structural atypia as those of invasive carcinomas.
In the diagnosis of adenocarcinoma arising from an adenoma, RK used "adenocarcinoma associated with adenoma" when there was good circumscription of the carcinoma from the surrounding or adjacent adenoma, which shows clearly different histology from the carcinoma (Fig. 8A). In cases showing adenoma with high-grade dysplasia with gradual transformation to carcinoma, all tumor components were categorized as adenocarcinoma (not carcinoma in adenoma). If tumor cell nuclei shared the same morphology in areas of both invasive adenocarcinoma and non-invasive tumor, diagnosis of adenocarcinoma was made by RK. However, KMK diagnosed adenocarcinoma arising in adenoma in cases harboring definite areas of adenoma and showing transformation into invasive adenocarcinoma (Fig. 8B).
Although interobserver variation was present, it was not extreme and didn't affect treatment plans. However, diagnosing carcinoma on the basis of cellular and structural atypia, such as is done in Japan, may lead to a higher prevalence of gastric carcinoma and relatively good therapeutic results. Further international studies would help pathologists improve poor interobserver agreement in the distinction between high-grade dysplasia and adenocarcinoma.
Figures and Tables
Fig. 1
Gastric biopsies diagnosed as erosion by both RK and KMK. Although the pit shows neutrophilic abscesses, there was no epithelial cell necrosis, suggesting erosion rather than neoplasia.
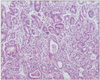
Fig. 2
Gastric biopsies diagnosed as suspected adenocarcinoma by RK. This patient was diagnosed with esophageal squamous cell carcinoma and had received chemo-radiation therapy for 3 months. KMK diagnosed this case as regenerative atypia.

Fig. 3
Gastric biopsies diagnosed as suspicious of adenocarcinoma by KMK. RK diagnosed this case as regenerative atypia because these cells contained Golgi areas in the subapical cytoplasm. KMK thought that those regenerative changes were caused by previous biopsy effects.
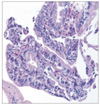
Fig. 5
Representative photomicrograph of a very well differentiated intramucosal intestinal type adenocarcinoma without invasion diagnosed by RK.
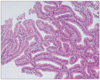
Fig. 6
Representative photomicrograph of an adenocarcinoma diagnosed by RK, but diagnosed as an adenoma with high-grade dysplasia by KMK.
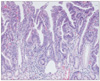
Fig. 7
Representative photomicrograph of gastric type adenocarcinoma diagnosed by RK, but diagnosed as adenoma with high-grade dysplasia by KMK.
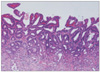
Fig. 8
Adenocarcinoma associated with adenoma diagnosed by RK (A) showing clearly different histology from carcinoma (arrow). Adenocarcinoma arising in adenoma diagnosed by KMK (B) showing a transition from adenoma to carcinoma (arrow).
Acknowledgments
We would like to thank Drs. Shimoda T and Park CK for their great support and advice.
References
1. Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000. 24:167–176.
2. Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001. 32:368–378.

3. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 2010. 4th ed. Lyon: World Health Organization;10–14.
4. Downs-Kelly E, Mendelin JE, Bennett AE, Castilla E, Henricks WH, Schoenfield L, et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett's esophagus biopsies. Am J Gastroenterol. 2008. 103:2333–2340.

5. Schlemper RJ, Itabashi M, Kato Y, Lewin KJ, Riddell RH, Shimoda T, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet. 1997. 349:1725–1729.

6. Kim JM, Cho MY, Sohn JH, Kang DY, Park CK, Kim WH, et al. Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Diagnosis of gastric epithelial neoplasia: Dilemma for Korean pathologists. World J Gastroenterol. 2011. 17:2602–2610.

7. Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000. 47:251–255.

8. Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003. 442:99–106.

9. Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005. 39:106–113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download