|
Infliximab |
|
|
|
|
|
|
|
|
|
|
|
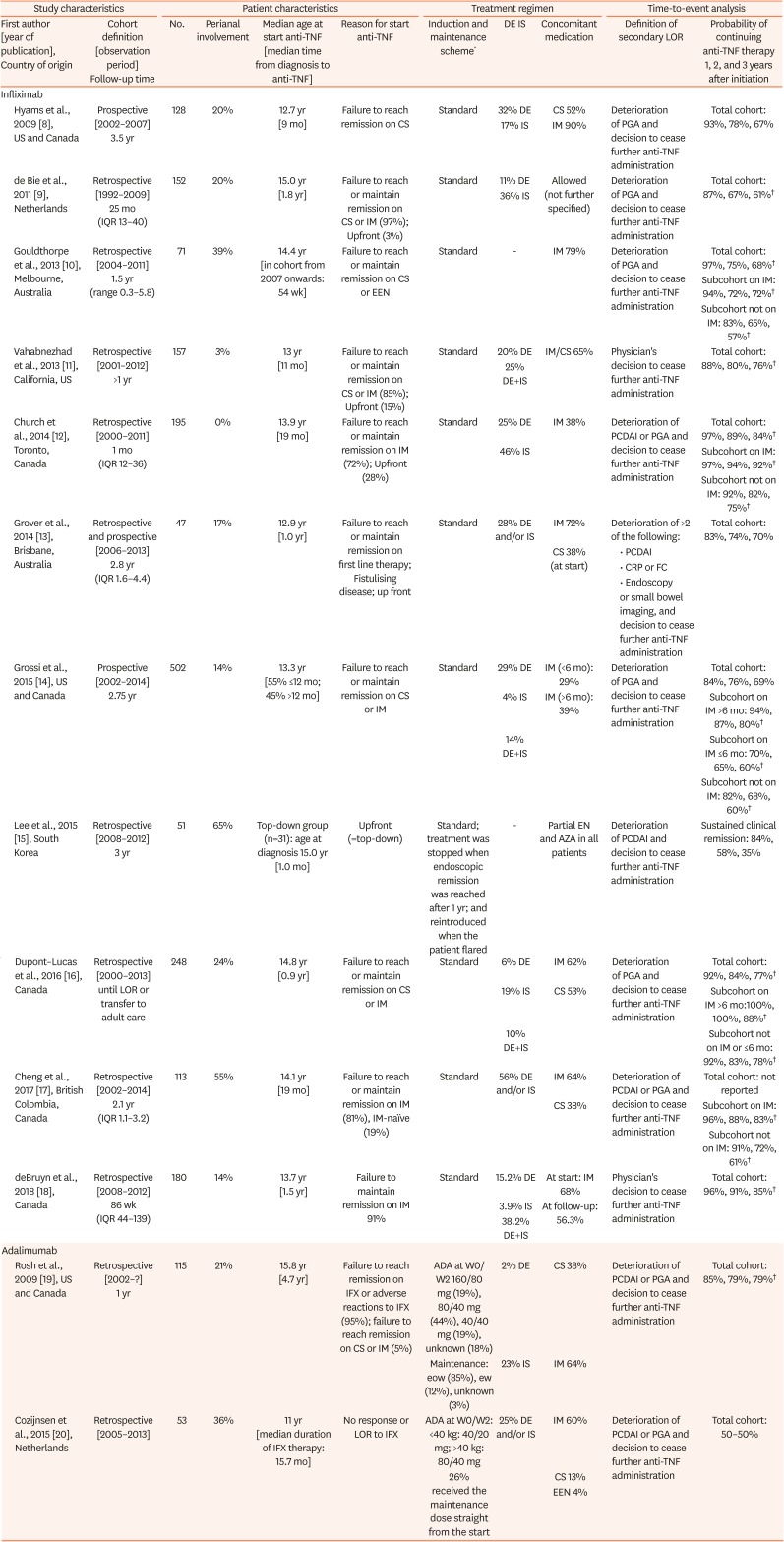
Hyams et al., 2009 [8], US and Canada |
Prospective [2002–2007] 3.5 yr |
128 |
20% |
12.7 yr [9 mo] |
Failure to reach remission on CS |
Standard |
32% DE |
CS 52% |
Deterioration of PGA and decision to cease further anti-TNF administration |
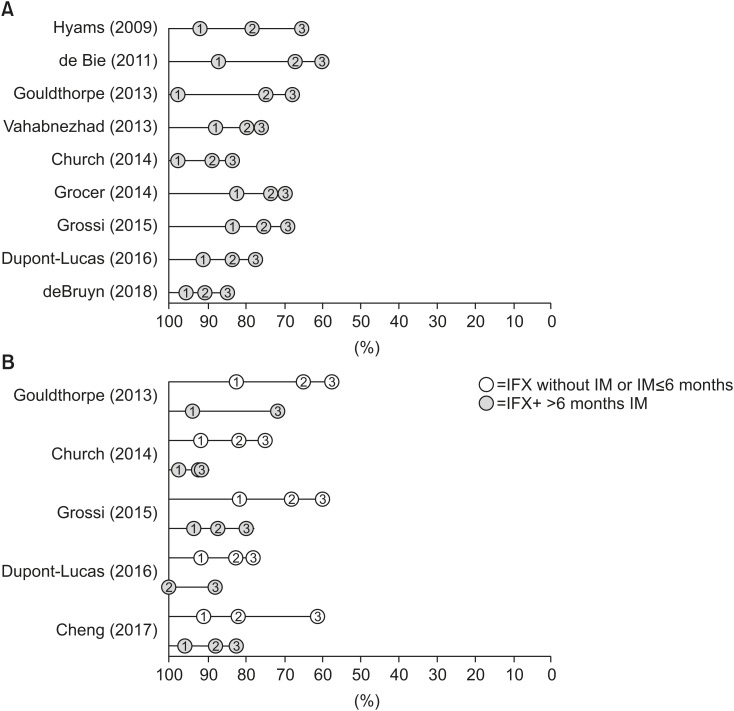
Total cohort: 93%, 78%, 67% |
|
17% IS |
IM 90% |
|
de Bie et al., 2011 [9], Netherlands |
Retrospective [1992–2009] 25 mo (IQR 13–40) |
152 |
20% |
15.0 yr [1.8 yr] |
Failure to reach or maintain remission on CS or IM (97%); Upfront (3%) |
Standard |
11% DE |
Allowed (not further specified) |
Deterioration of PGA and decision to cease further anti-TNF administration |
Total cohort: 87%, 67%, 61%†
|
|
36% IS |
|
Gouldthorpe et al., 2013 [10], Melbourne, Australia |
Retrospective [2004–2011] 1.5 yr (range 0.3–5.8) |
71 |
39% |
14.4 yr [in cohort from 2007 onwards: 54 wk] |
Failure to reach or maintain remission on CS or EEN |
Standard |
- |
IM 79% |
Deterioration of PGA and decision to cease further anti-TNF administration |
Total cohort: 97%, 75%, 68%†
|
|
Subcohort on IM: 94%, 72%, 72%†
|
|
Subcohort not on IM: 83%, 65%, 57%†
|
|
Vahabnezhad et al., 2013 [11], California, US |
Retrospective [2001–2012] >1 yr |
157 |
3% |
13 yr [11 mo] |
Failure to reach or maintain remission on CS or IM (85%); Upfront (15%) |
Standard |
20% DE |
IM/CS 65% |
Physician's decision to cease further anti-TNF administration |
Total cohort: 88%, 80%, 76%†
|
|
25% DE+IS |
|
Church et al., 2014 [12], Toronto, Canada |
Retrospective [2000–2011] 1 mo (IQR 12–36) |
195 |
0% |
13.9 yr [19 mo] |
Failure to reach or maintain remission on IM (72%); Upfront (28%) |
Standard |
25% DE |
IM 38% |
Deterioration of PCDAI or PGA and decision to cease further anti-TNF administration |
Total cohort: 97%, 89%, 84%†
|
|
46% IS |
Subcohort on IM: 97%, 94%, 92%†
|
|
Subcohort not on IM: 92%, 82%, 75%†
|
|
Grover et al., 2014 [13], Brisbane, Australia |
Retrospective and prospective [2006–2013] 2.8 yr (IQR 1.6–4.4) |
47 |
17% |
12.9 yr [1.0 yr] |
Failure to reach or maintain remission on first line therapy; Fistulising disease; up front |
Standard |
28% DE and/or IS |
IM 72% |
Deterioration of >2 of the following: |
Total cohort: 83%, 74%, 70% |
|
CS 38% (at start) |
|
• PCDAI |
|
• CRP or FC |
|
• Endoscopy |
|
or small bowel imaging, and decision to cease further anti-TNF administration |
|
Grossi et al., 2015 [14], US and Canada |
Prospective [2002–2014] 2.75 yr |
502 |
14% |
13.3 yr [55% ≤12 mo; 45% >12 mo] |
Failure to reach or maintain remission on CS or IM |
Standard |
29% DE |
IM (<6 mo): 29% |
Deterioration of PGA and decision to cease further anti-TNF administration |
Total cohort: 84%, 76%, 69% |
|
4% IS |
IM (>6 mo): 39% |
Subcohort on IM >6 mo: 94%, 87%, 80%†
|
|
14% DE+IS |
Subcohort on IM ≤6 mo: 70%, 65%, 60%†
|
|
Subcohort not on IM: 82%, 68%, 60%†
|
|
Lee et al., 2015 [15], South Korea |
Retrospective [2008–2012] 3 yr |
51 |
65% |
Top-down group (n=31): age at diagnosis 15.0 yr [1.0 mo] |
Upfront (=top-down) |
Standard; treatment was stopped when endoscopic remission was reached after 1 yr; and reintroduced when the patient flared |
- |
Partial EN and AZA in all patients |
Deterioration of PCDAI and decision to cease further anti-TNF administration |
Sustained clinical remission: 84%, 58%, 35% |
|
Dupont–Lucas et al., 2016 [16], Canada |
Retrospective [2000–2013] until LOR or transfer to adult care |
248 |
24% |
14.8 yr [0.9 yr] |
Failure to reach or maintain remission on CS or IM |
Standard |
6% DE |
IM 62% |
Deterioration of PGA and decision to cease further anti-TNF administration |
Total cohort: 92%, 84%, 77%†
|
|
19% IS |
CS 53% |
Subcohort on IM >6 mo:100%, 100%, 88%†
|
|
10% DE+IS |
Subcohort not on IM or ≤6 mo: 92%, 83%, 78%†
|
|
Cheng et al., 2017 [17], British Colombia, Canada |
Retrospective [2002–2014] 2.1 yr (IQR 1.1–3.2) |
113 |
55% |
14.1 yr [19 mo] |
Failure to reach or maintain remission on IM (81%), IM-naïve (19%) |
Standard |
56% DE and/or IS |
IM 64% |
Deterioration of PCDAI or PGA and decision to cease further anti-TNF administration |
Total cohort: not reported |
|
CS 38% |
Subcohort on IM: 96%, 88%, 83%†
|
|
Subcohort not on IM: 91%, 72%, 61%†
|
|
deBruyn et al., 2018 [18], Canada |
Retrospective [2008–2012] 86 wk (IQR 44–139) |
180 |
14% |
13.7 yr [1.5 yr] |
Failure to maintain remission on IM 91% |
Standard |
15.2% DE |
At start: IM 68% |
Physician's decision to cease further anti-TNF administration |
Total cohort: 96%, 91%, 85%†
|
|
3.9% IS |
At follow-up: 56.3% |
|
38.2% DE+IS |
|
Adalimumab |
|
|
|
|
|
|
|
|
|
|
|
|
Rosh et al., 2009 [19], US and Canada |
Retrospective [2002–?] 1 yr |
115 |
21% |
15.8 yr [4.7 yr] |
Failure to reach remission on IFX or adverse reactions to IFX (95%); failure to reach remission on CS or IM (5%) |
ADA at W0/W2 160/80 mg (19%), 80/40 mg (44%), 40/40 mg (19%), unknown (18%) |
2% DE |
CS 38% |
Deterioration of PCDAI or PGA and decision to cease further anti-TNF administration |
Total cohort: 85%, 79%, 79%†
|
|
Maintenance: eow (85%), ew (12%), unknown (3%) |
23% IS |
IM 64% |
|
Cozijnsen et al., 2015 [20], Netherlands |
Retrospective [2005–2013] |
53 |
36% |
11 yr [median duration of IFX therapy: 15.7 mo] |
No response or LOR to IFX |
ADA at W0/W2: <40 kg: 40/20 mg; >40 kg: 80/40 mg |
25% DE and/or IS |
IM 60% |
Deterioration of PCDAI or PGA and decision to cease further anti-TNF administration |
Total cohort: 50–50% |
|
26% received the maintenance dose straight from the start |
CS 13% |
|
EEN 4% |





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download