This article has been
cited by other articles in ScienceCentral.
Abstract
The incidence of acute pancreatitis (AP) has increased in the pediatric population over the past few decades and it stands to follow that the complications of severe AP, including symptomatic pancreatic fluid collections (PFCs) will increase as well. In adults, the therapeutic options for this situation have undergone a dramatic evolution from mainly surgical approaches to less invasive endoscopic approaches, mainly endoscopic ultrasound-guided transmural drainage (EUS-TD) followed be direct endoscopic necrosectomy if needed. This has proven safe and effective in adults; however, this approach has not been well studied or reported in pediatric populations. Here we demonstrate that EUS-TD seems to offer a safe, efficacious and minimally invasive approach to the management of large PFCs in pediatric patients by reviewing two representative cases at our institution.
Keywords: Acute pancreatic fluid collection, Endoscopic ultrasound-guided transmural drainage
INTRODUCTION
Pancreatic fluid collections (PFCs) are common sequalae which develop as a complication of severe acute pancreatitis (AP). The incidence of AP has increased in the pediatric population, and complications such as PFCs are not uncommon [
12]. Asymptomatic or small PFCs can typically be managed with conservative therapy and no intervention, however; more severe cases often warrant intervention for PFC drainage. PFCs as a result of AP in pediatric patients have been historically managed by surgery or percutaneous drainage, however, surgery is associated with significant morbidity and percutaneous approaches cannot effectively remove solid debris and may lead to injury or pancreaticocutaneous fistula. endoscopic ultrasound-guided transmural drainage (EUS-TD) of PFCs in adults is evidence-based and has become commonplace with good clinical efficacy and a superior safety profile when compared to surgery (complication rates 10% vs. up to 30%, respectively) [
3]. In the pediatric population, however, endoscopic management has not been well studied, notably in large fluid collections.
Here we demonstrate that EUS-TD seems to offer a safe, efficacious and minimally invasive treatment modality for large PFCs in appropriately selected pediatric patients, similar to adults. We review two patients with PFCs who successfully underwent EUS-TD at our institution.
CASE REPORT
Case 1
A 2-year-old female with high-risk B-cell acute lymphoblastic leukemia (ALL) developed severe AP after being treated with induction therapy. At presentation, she was afebrile and tachycardic. Labs revealed a serum amylase of 482 U/L and lipase >4,000 U/L. Following conservative treatment with intravenous fluid resuscitation and bowel rest, her symptoms resolved; however, three weeks later, she developed worsening abdominal pain, multiple episodes of nonbilious emesis, and an up-trending serum lipase. Abdominal ultrasound and magnetic resonance cholangiopancreatography revealed an acute pancreatic fluid collection measuring 9.8×7.2×10.2 cm with no evidence of pancreatic necrosis nor obstruction of the pancreatic or biliary ducts (
Fig. 1A). After four weeks of conservative management, serial imaging showed gradual increases in size of the complex fluid collections involving the retroperitoneum and extending from the diaphragm to the left lobe of the liver measuring 12 cm across with a second adjacent collection in the left upper quadrant lying above the spleen. One week later, due to persistent epigastric pain, nausea, vomiting, it was felt that endoscopic drainage of the PFC was indicated for definitive treatment.
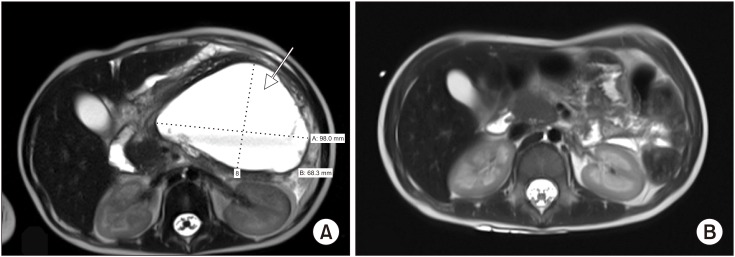
Fig. 1
(A) T2-weighted magnetic resonance imaging (MRI) showing an acute peripancreatic fluid collection measuring 9.8×7.2×10.2 cm (arrow) prior to endoscopic ultrasound-guided transmural drainage. (B) Follow-up MRI at 5 months showing resolution of the pancreatic fluid collection.
A pediatric linear EUS videoendoscope (Olympus CF-2T160L; Olympus, Southborough, MA, USA) was used to perform EUS-TD. Once the fluid collection abutting the gastric wall was visualized by EUS, a 19-gauge needle was used to puncture the gastric wall through a Doppler free window. A 0.035 g guidewire was then introduced to allow for CRE balloon dilation of the transgastric access to 13.5 mm. Following dilation, a small leak of air was observed on distension of the stomach and under the liver concerning for perforation at the ostomy site. Further inspection noted dehiscence of the stomach from the cyst wall which was repaired using five endoscopic clips approximating the cyst wall to the gastric wall. To ensure adequate closure, contrast dye was injected, which revealed a proper repair. Two double-pigtail stents (10 Fr×10 cm and 7 Fr×10 cm) were then introduced over the guidewire to allow for continuous drainage. At the end of the procedure, a nasogastric tube was placed for decompression and broad-spectrum antibiotics were given to treat empirically for peritonitis. The patient tolerated the procedure well. Since she was receiving induction chemotherapy, she remained in the hospital post-operatively. Serial ultrasound imaging revealed interval decreases in the size of the PFC and her abdominal pain resolved. The stents were removed one month and five months post operatively and follow-up magnetic resonance imaging (MRI) showed resolution of the fluid collection (
Fig. 1B). She is currently doing very well, symptom free, and over three years status post treatment for her high-risk B-cell ALL.
Case 2
A 4-year-old male with no significant medical history presented as a transfer from an outside hospital due to symptoms of nausea and postprandial, nonbilious emesis and abdominal imaging consistent with an acute peripancreatic fluid collection (APFC) in the setting of AP. At the outside emergency department, non-contrast abdominal CT imaging revealed a 7.5×5.5×6.3 cm, well-circumscribed fluid collection anterior and superior to the pancreas with associated peripancreatic fluid, with history concerning for blunt abdominal trauma due to abuse.
Upon presentation at our institution, he was febrile at 38.7C and tachycardic. He had a mild leukocytosis and an elevated lipase of 196 U/L. Physical examination was significant for a comfortable although thin and mildly malnourished four-year old male with mild abdominal distension, tenderness to palpation in the epigastric region and bruising of his left hand, wrist and right foot and also evidence of prior skin burns. He was started on broad-spectrum antibiotics, intravenous fluids and bowel rest with nasojejeunal tube feeds for seven days.
X-ray of the upper extremities revealed multiple fractures in various stages of healing, including healing proximal phalangeal fractures and a nondisplaced fracture of the seventh rib. These constellations of findings were suggestive that intentional injury and blunt abdominal trauma had led to the development of AP and APFCs.
MRI of the abdomen without contrast showed an enlarging pancreatic fluid collection measuring 10.8×6.6×10.4 cm (
Fig. 2A). Due to the interval increase in the size of the APFC, EUS-TD with stent placement was performed.
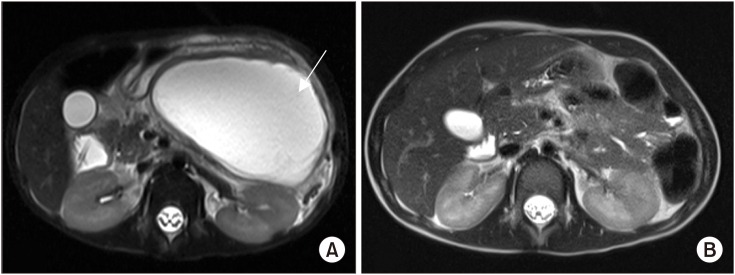
Fig. 2
(A) T2-weighted magnetic resonance imaging (MRI) showing an acute pancreatic fluid collection measuring 10.8×6.6×10.4 cm (arrow) prior to endoscopic ultrasound-guided transmural drainage. (B) Repeat MRI at four weeks showing resolution of the pancreatic fluid collection.
The PFC was accessed under EUS-guidance and an AXIOS (Xlueman Inc., Mountain View, CA, USA) stent was placed under EUS guidance (
Fig. 3). The cystogastrostomy was dilated with a controlled radial expansion balloon to 15 mm and over 1L of fluid was drained. An additional 5 cm soft double pigtail stent was placed to minimize trauma from the distal end of the AXIOS stent.
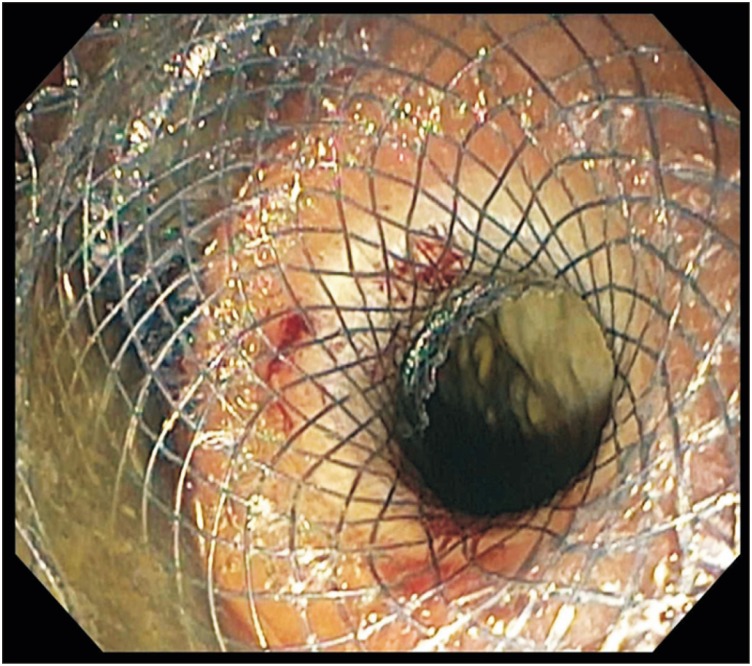
Fig. 3
Endoscopic image showing deployment of an AXIOS stent for endoscopic ultrasound-guided transmural drainage.
The patient tolerated the procedure well and symptoms improved after the procedure. His diet was advanced and he was discharged with close follow-up. Repeat MRI four weeks post-procedure revealed resolution of the PFC (
Fig. 2B) with pigtail drain still in place. Three months following EUS-TD, the stent was removed from the cystogastrostomy and ultrasound nine months post-procedure revealed no evidence of recurrence of the PFC.
DISCUSSION
PFCs arise as sequalae of AP in up to 58.6% of cases in pediatric populations [
4]. The revised Atlanta criteria classifies PFCs as acute or chronic, with acute occurring less than 4 weeks and chronic greater than 4 weeks from the initial episode of acute AP [
5]. Within four weeks of onset of AP, PFCs are subdivided into APFCs or acute necrotic fluid collections (which have significant solid debris). After four weeks following the onset of AP, PFCs are classified into a pancreatic pseudocyst or walled-off pancreatic necrosis. Symptomatic PFCs require drainage, with options including surgical, percutaneous, and endoscopic approaches. In the adult population, endoscopic ultrasound with transluminal stent placement with then direct endoscopic necrosectomy to remove significant solid debris has largely replaced surgical approaches due to its superior safety profile, feasibility, and efficacy. A 2013 randomized trial compared the efficacy of endoscopic versus surgical cystogastrostomy in pancreatic pseudocyst drainage and found that endoscopic technique is noninferior to surgical drainage, with similar rates of complications, and reinterventions [
6].
There is limited data regarding endoscopic management of PFCs in the pediatric population, yet there is an emerging role of EUS as a diagnostic and therapeutic modality in pediatric pancreatobiliary disorders [
7]. The first reported conventional endoscopic internal drainage of a pediatric pancreatic fluid collection without EUS was in 1996, and since then, there has been some evidence and case reports in the literature that supports adoption of this technique as a successful alternative to surgery [
58].
EUS-guided transmural drainage adds to the safety and efficacy of the drainage procedure as intervening vessels can be visualized and avoided during puncture of the PFC. In the adult population, complication rates of EUS-TD are reported to be 5% for drainage of uninfected PFCs vs as high and 30% in surgical drainage [
37]. This minimally invasive approach offers good efficacy and a superior safety profile to other drainage modalities in adults. Here we demonstrate two cases where large PFCs were successfully managed with EUS-TD with no reported adverse events. In the second case, we demonstrate the use of a fully-covered, self-expandable AXIOS metallic stent (Xlumena Inc., Mountain View, CA, USA) with dually-anchored flanges that provide stability, minimized risk of migration due to its anchoring effect, and maintenance of a large SEMS lumen for adequate drainage.
This two patient case series emphasizes that EUS-TD of PFCs in the pediatric population may provide equal efficacy to open drainage approaches, with reduced morbidity in a population in desperate need of improved treatment options. More robust clinical trials will be required to confirm these results and further develop this approach.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download