Abstract
Recently, fecal microbiota transplantation (FMT) has been attracting attention as a possible medical treatment of ulcerative colitis (UC). A randomized controlled trial of FMT for children with UC is currently underway. Therapeutic effects of FMT for adults with UC remain controversial. We report two cases of early-onset UC in children. A patient was diagnosed with UC at age 1-year 9-month and underwent FMT at age 2-year 3-month. He attained clinical remission for three weeks after FMT, but then relapsed at four weeks, ultimately undergoing a total colectomy. Another child was diagnosed with UC at 2-year 10-month and she underwent FMT at age 5 years. She has remained in clinical remission following FMT for 24 months and her UC has been maintained without complications with tacrolimus and azathioprine. We report that FMT for early-onset UC appears to be safe and potentially effective.
While prevalence of inflammatory bowel disease (IBD) in Europe and North America has remained constant, incidence of ulcerative colitis (UC) in Japan is rising [1]. From 1991–2014, prevalence of UC in Japan increased 7.4-fold from 18.1 to 133.3 per 100,000 persons [1,2,3] and the total number of patients with UC in Japan is now the second largest in the world. This recent increase in Japan also includes children with UC [4].
Compared with adults, UC in children characteristically affects the entire colon and is often more severe [5]. Onset of IBD before age 6 years is referred to as very early onset IBD (VEO-IBD), and such cases have been reported to be particularly severe [6]. Similar to adults with UC, colectomy is indicated for pediatric patients with UC unresponsive to medical treatment. Complications and adverse effects of steroids and immunosuppressive agents, growth disturbance, and reduced child-specific quality of life should be considered when considering total colectomy for children. In contrast to Crohn's disease, some pediatricians may consider UC to be curable if total colectomy and ileal pouch-anal anastomosis (IPAA) are performed. However, it has been reported that an idiopathic non-specific form of inflammation, referred to as pouchitis and/or pre-pouch ileitis, develops postoperatively, and in recent years, incidence of such postoperative inflammation has been approximately 60% [7,8]. So, except for cases in which surgery is necessary, attempts at medical management should be made.
Recently, fecal microbiota transplantation (FMT) has been attracting attention as a potentially promising medical treatment of UC. While therapeutic effects of FMT for UC remain controversial, a recent randomized controlled trial (RCT) in adults revealed that FMT was effective compared with placebo [9]. An RCT of FMT for children with UC is currently underway, although administration route and administration method are inconsistent between institutions [10]. To date, there have been no RCT reports of FMT for UC in young children, which would be VEO-IBD [11].
In this report, we present our experience of FMT in two patients with VEO-ulcerative colitis (VEO-UC). In both patients, active UC was uncontrollable by either conventional medical treatment, including immunosuppressive agents such as azathioprine and cyclosporine, or by infliximab. Both patients underwent FMT as a last resort before surgery. In case 1, temporary clinical remission was observed for three weeks following FMT; however, the UC flared up in the fourth week, and the patient ultimately underwent surgery. In case 2, fecal analysis three months after FMT revealed intestinal bacterial flora that was similar to that in the stool of the donor, that led to sustained clinical remission of UC activity for the past two years.
Candidates for FMT include patients with recurrence that are steroid-dependent or resistant, patients in whom medical treatment does not result in clinical remission or causes complications and adverse effects, and patients for whom there is no other effective alternative non-surgical medical treatment. Candidates were evaluated and approved by our Institutional Review Board (IRB number: 29-50).
Donor selection criteria included up to second-degree relatives. Exclusion criteria are presented in Table 1. Fecal donors, even minors, received explanations from researchers and signed an authorization and consent document. Screening costs for a donor were entirely at the expense of the patient's family.
Sterilization of instruments was performed using an autoclave. Sterile gloves and gown were worn, and processing of the sample was performed with sanitizing procedures to prevent contamination with other microbes. Approximately 100 g of the collected donor stool was mixed with sterile saline (200–250 mL), heated to 37℃, and then filtered within three hours.
The number of FMT administrations was set at one per day for five consecutive days. As pretreatment, colonic irrigation using a laxative was performed the day prior to FMT. Hypertonic magnesium citrate liquid (Magcorol P®, Horii Pharma, Osaka, Japan) was used as the laxative in accordance with package inserts. With regard to the administration route, we consulted both sets of parents chose either intracolonic administration into the cecum via colonoscopy (CS) or administration beyond the duodenum after insertion of an enteral feeding tube via the esophagus (esophago-duodenal [ED] tube).
The patient was a 2-year 3-month old Japanese male diagnosed with UC affecting the entire colon at age 1 year 9 months. On whole-exome sequencing analysis, known congenital immunodeficiency and chronic diarrhea were ruled out. After onset, repeated flare-ups of refractory UC occurred. Remission could not be induced despite fasting, total parenteral nutrition, azathioprine administration, continuous intravenous cyclosporine infusion, and infliximab administration. Consequently, with the strong desire of both parents, FMT was performed. The mother was selected to be the donor, and using CS, an average of 100 g of donor stool was transplanted daily for five consecutive days. Prior to FMT, the patient had a PUCAI score of 35 points (Fig. 1A), indicating moderate activity of UC. In the evaluation performed seven days after treatment, the Pediatric Ulcerative Colitis Activity Index (PUCAI) score reduced to 5 points. It then further reduced to 0 points in the evaluation performed two weeks after treatment (Fig. 1B). Clinical remission persisted for three weeks after treatment and CS revealed an improvement in mucosa lesions. However, in the fourth week after treatment, the UC flared up again with a PUCAI score of 35 points. Thus, at eight weeks after FMT, the patient underwent total colectomy and IPAA. Pathological findings of the removed large intestine demonstrated inflammation and diffuse fresh bleeding, caused by UC flare up. No granulomas or cytomegalovirus were detected.
The patient was a 5-year-old Japanese female diagnosed with UC affecting the entire colon at age 2 years 10 months. There were no detectable genes on whole-exome sequencing analysis. During the ensuing year she was hospitalized 14 times, with remission finally maintained by the administration of azathioprine, tacrolimus at a dose of 0.3 µg/kg/day, and infliximab therapy. However, at age 4 years, she developed acute kidney injury caused by tacrolimus, upon reducing tacrolimus dose to 0.1 mg/kg/day, a severe UC flare-up occurred with a PUCAI score of 75 points. Tacrolimus was increased again to 0.3 µg/kg/day, that reduced the PUCAI score to 15 points; however, re-exacerbation of the acute kidney injury led to consideration of total colectomy. The parents desired FMT for the patient; thus, at age 5 years, FMT was performed for five consecutive days using an ED tube. The patient's 13-year-old brother served as the donor. Along with his parent's consent form, the minor's consent document was approved by the IRB. One week after FMT, clinical remission was achieved with a PUCAI score of 0 points. On fecal analysis at 3 months after FMT, intestinal bacterial flora was similar to that present in the stool of the donor (Fig. 2). At present, 24 months after FMT, the patient is age 7 years, and remission of UC has been maintained by administration of tacrolimus at a dose of 0.2 µg/kg/day and azathioprine at 2.5 mg/kg/day.
We performed FMT in two patients with VEO-UC that could not be controlled by conventional non-surgical medical treatment and we highlight three important points. First, in patients with VEO-UC resistant to current medical treatment options, FMT may potentially induce temporal or even longer term clinical remission. Second, the administration route may not have had an impact on the effect of the FMT in our cases. Third, there were no severe complications of FMT in our cases.
The first observation of this report was that FMT in patients with VEO-UC may lead to long-term and short-term clinical remission. The purpose of FMT is to first induce remission of UC, and then to subsequently maintain long-term remission. In one of our patients, FMT successfully resulted in long-term clinical remission, which at the time of this report, has been successfully maintained for 24 months. When checked at the three-month period after treatment, the recipient's intestinal bacterial flora had changed to approximately that of the donor's bacterial flora. Performing FMT is worthwhile, even if clinical remission is only short term, as this may allow the patient's general condition to stabilize so that surgery can be undertaken safely. At our hospital, FMT is indicated for patients with flare-ups dependent on or resistant to steroids, for those with refractory UC that do not go into remission despite introduction of calcineurin preparations or biological preparations, and candidates for surgical treatment. For our patient that revealed only a short-term clinical remission following FMT, pre-transplant clinical remission had never been achieved; neither through pharmacotherapy nor leukocytopheresis. Because this patient suffered from recurring episodes of severe bloody stools, abdominal pain, and diarrhea, the patient and the patient's family were emotionally unsettled. However, clinical symptoms improved after FMT, that physically and emotionally stabilized the patient. Even though long-term clinical remission post-FMT was not maintained, we safely proceeded with surgery. There were no severe complications of FMT observed in either of our two patients.
The second observation of our study was that administration route of FMT may not make a difference in the post-procedure effects seen in patients with VEO-UC. Among our two patients, the patient that revealed long-term remission underwent FMT via ED tube into the small intestine and the patient that revealed short-term remission received FMT via the colon using CS. Looking at other children in Japan that had previously undergone FMT, a 3-year-old girl that received FMT via extended enema (twice) and via the small intestine (four times) did not go into remission and required total colectomy [13]. In an 11-year-old female that received FMT via the colon, remission was successfully achieved and continues to be maintained [12]. FMT for children with UC is most commonly administered into the colon [10]. Rationale for administration via this route is that, since inflammation in UC develops only in the colon, FMT delivery into the small intestines should not be required. We must not forget, though, that since most patients with VEO-UC present with UC affecting the entire colon, it is possible that the cleansing effect of pre-transplant enema may not reach all the way to the right hemi-colon. To fully diffuse the effect of FMT using CS, the cecum must be reached. Performing CS each time on infants is a challenging task. So, we believe that for UC affecting the entire colon, administration via the small intestines through an ED tube is a more appropriate administration route.
The third observation of our study was that FMT was performed safely in both of our cases of VEO-UC and did not cause severe complications. No major complications have been observed in past reports of FMT in Japan [12,13]; however, reports from other countries have noted cytomegalovirus enteritis and Clostridium difficile infection associated with collected donor stool [14], and systemic complications such as bacteremia have been reported in cases of FMT in children [15]. It is important to monitor carefully after implementing FMT.
We safely performed FMT in two patients with VEO-UC, and in one patient, long-term clinical remission was achieved. Even in patients in whom long-term remission cannot be achieved, a period of short-term clinical remission may be accomplished that can provide additional time for improvement in general condition prior to necessary surgery. To improve the therapeutic effect of FMT, further evaluation should be performed with regard to selection of FMT candidate patients, optimal administration route, number of administrations, and donor selection.
Figures and Tables
Fig. 1
The endoscopic findings of case 1. Transverse colon (A) before fecal microbiota transplantation (FMT), and (B) at one week after starting FMT. (A) demonstrated severe diffuse and continuous mucosal inflammation with erythema and bleeding, whereas (B) demonstrated improved erythema and vascular pattern.
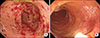
Fig. 2
Fecal microbiota composition of the recipient and donor measured by T-RFLP (terminal restriction fragments length polymorphism). Before fecal microbiota transplantation (FMT), Bacteroides fragilis comprised more than two-thirds of the patient's microbiota. The graphs demonstrate that the patient's microbiota composition was quite different from the donor. However, fecal analysis three months after FMT revealed intestinal bacterial flora that was similar to that present in the stool of the donor, that led to clinical remission of ulcerative colitis activity for the subsequent two years.

ACKNOWLEDGEMENTS
We thank Drs. Iwama Itaru, Kei Matayoshi, Taisuke Tsuji, and Saori Kinjo for the patients' care.
References
1. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn's disease in Japan. J Gastroenterol. 2009; 44:659–665.

2. Morita N, Toki S, Hirohashi T, Minoda T, Ogawa K, Kono S, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995; 30:Suppl 8. 1–4.
3. Japanese Ministry of Health, Labour and Welfare. Handbook of health and welfare statistics 2016 contents. Table 2-22 number of persons with specific (intractable) disease healthcare certificate by disease and sex [Internet]. Tokyo: Japanese Ministry of Health, Labour and Welfare;2018. cited 2018 Jan 15. Available from: http://www.mhlw.go.jp/english/database/db-hh/2-1.html.
4. Ishige T, Tomomasa T, Takebayashi T, Asakura K, Watanabe M, Suzuki T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. 2010; 45:911–917.

5. Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014; 147:990–1007.

6. Kelsen J, Sullivan K. Immune dysregulation associated with very early-onset inflammatory bowel disease. In : Mamula P, Grossman AB, Baldassano RN, Kelsen JR, Markowitz JE, editors. Pediatric inflammatory bowel disease. 3rd ed. Switzerland: Springer;2017. p. 55–67.
7. Rinawi F, Assa A, Eliakim R, Mozer Glassberg Y, Nachmias Friedler V, Niv Y, et al. Predictors of pouchitis after ileal pouch-anal anastomosis in pediatric-onset ulcerative colitis. Eur J Gastroenterol Hepatol. 2017; 29:1079–1085.

8. Dharmaraj R, Dasgupta M, Simpson P, Noe J. Predictors of pouchitis after Ileal pouch-anal anastomosis in children. J Pediatr Gastroenterol Nutr. 2016; 63:e58–e62.

9. Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.

10. Pai N, Popov J. Protocol for a randomised, placebo-controlled pilot study for assessing feasibility and efficacy of faecal microbiota transplantation in a paediatric ulcerative colitis population: PediFETCh trial. BMJ Open. 2017; 7:e016698.

11. Hourigan SK, Oliva-Hemker M. Fecal microbiota transplantation in children: a brief review. Pediatr Res. 2016; 80:2–6.

12. Shimizu H, Arai K, Abe J, Nakabayashi K, Yoshioka T, Hosoi K, et al. Repeated fecal microbiota transplantation in a child with ulcerative colitis. Pediatr Int. 2016; 58:781–785.

13. Kumagai H, Yokoyama K, Imagawa T, Inoue S, Tulyeu J, Tanaka M, et al. Failure of fecal microbiota transplantation in a three-year-old child with severe refractory ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr. 2016; 19:214–220.

14. Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016; 11:e0161174.

15. Vandenplas Y, Veereman G, van der Werff Ten Bosch J, Goossens A, Pierard D, Samsom JN, et al. Fecal microbial transplantation in early-onset colitis: caution advised. J Pediatr Gastroenterol Nutr. 2015; 61:e12–e14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download