Abstract
Epstein-Barr virus (EBV) infection can be presented with various clinical manifestations and different levels of severity when infected. Infectious mononucleosis, which is most commonly caused by EBV infection in children and adolescents, is a clinical syndrome characterized by fatigue, malaise, fever, sore throat, and generalized lymphadenopathy. But rarely, patients with infectious mononucleosis may present with gastrointestinal symptoms and complicated by gastritis, splenic infarction, and splenic rupture. We encountered a 16-year-old girl who presented with fever, fatigue, and epigastric pain. Splenic infarction and EBV-associated gastritis were diagnosed by using esophagogastroduodenoscopy and abdominal computed tomography. Endoscopy revealed a generalized hyperemic nodular lesion in the stomach, and the biopsy findings were chronic gastritis with erosion and positive in situ hybridization for EBV. As splenic infarction and acute gastritis are rare in infectious mononucleosis and are prone to be overlooked, we must consider these complications when an infectious mononucleosis patient presents with gastrointestinal symptom.
The Epstein-Barr virus (EBV), a member of the gamma-herpes virus, consists of a 172-kb double-stranded DNA [1]. Infectious mononucleosis is a clinical syndrome, consisting of fever, pharyngitis, and generalized lymphadenopathy [2]. Most cases of infectious mononucleosis are self-limiting diseases recovered by conservative treatment alone and rarely associated with complication. However, in rare cases, infectious mononucleosis can be associated with gastrointestinal tract symptoms, and can cause complications such a gastritis, splenic infarction, splenic rupture, lymphoma, and gastric cancer [34]. Because splenic rupture, lymphoma, and gastric cancer may be life threatening, careful observation is required when patients with gastrointestinal symptoms are suspected of infectious mononucleosis. Patients with acute gastritis or splenic infarction in infectious mononucleosis have been reported in some cases but patients with both complications simultaneously have not been reported yet. Herein, we report a case of EBV-associated infectious mononucleosis complicated by acute gastritis and splenic infarction in a 16-year-old girl.
A 16-year-old girl was hospitalized in the emergency department with an 8-day history of fever and fatigue and a 3-day history of epigastric pain. The patient had no symptoms of vomiting, diarrhea, or weight loss. In addition, the patient had no history of liver disease or use of herbal medicines.
A physical examination revealed a body temperature of 38.4℃, blood pressure of 126/84 mmHg, heart rate of 132 beats/min, and respiratory rate of 26 breaths/min. Pharyngeal injection and tonsilar hypertrophy grade II was observed. Hard, mobile and mildly tender lymph nodes measuring 4×2 cm were noted on the right neck level II. The abdomen was mildly distended and hard on palpation. Epigastric area and right lower quadrant tenderness were noticed with normal bowel sounds. The liver was palpable 5 cm below the right low costal margin, and the spleen was palpable 2 finger-breaths below the left costal margin.
The cell blood count examination revealed the following parameters: white blood cell count, 26,520/mm3 (segmental neutrophils, 12%; lymphocytes, 80%; and atypical lymphocytes, 37%); hemoglobin level, 12.6 g/dL; hematocrit, 37.4%; and platelet count, 190,000/mm3. The liver function tests revealed aspartate transaminase (AST), 253 IU/L; alanine transaminase (ALT), 326 IU/L; alkaline phosphatase (ALP), 197 IU/L; γ-glutamyl transpeptidase (γ-GTP), 333 IU/L; and serum lactate dehydrogenase (LDH), 636 IU/L which were highly elevated above normal range. Other serum chemistries were performed and the results were as follows: total bilirubin, 1.06 mg/dL; total protein, 6.4 g/dL; albumin, 3.3 g/dL; blood urea nitrogen, 7.7 mg/dL; creatinine, 0.7 mg/dL; and C-reactive protein, 0.47 mg/dL which were within normal range. The blood coagulation tests revealed prothrombin time (PT) 12.6; and activated partial thromboplastin time (aPTT) 40.1 seconds. The serologic tests for hepatitis virus strains A, B, and C were negative. The serum showed positivity for immunoglobulin (Ig)M antibody against EBV viral capsid antigen (EBV-VCA), and polymerase chain reaction (PCR) in peripheral whole blood was positive for EBV DNA, with a viral load of 10,828 copies/mL.
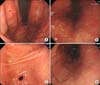
Abdominal and pelvic computed tomography (CT) showed hepatosplenomegaly, 2×1.6 cm low attenuated lesion that was consistent with splenic infarction, periportal edema and diffuse gallbladder wall thickening, and multiple slightly enlarged lymph nodes around aorta and mesentery. It was also possible to observe liver periportal edema and diffuse gallbladder wall thickening, multiple enlarged lymph nodes around the aorta and mesentery, and the large amount of ascites in pelvic cavity, all of which suggested lymphoma more likely than infectious disease (Fig. 1A, B). Esophagogastroduodenoscopy (EGD) was performed to evaluate the epigastric pain; EGD revealed a generalized hyperemic nodular lesion in the stomach (Fig. 2A, B). The biopsy findings were chronic gastritis with erosion and positive in situ hybridization for EBV (Fig. 3). The Warthin-Starry stain for Helicobacter pylori was negative. For differential diagnosis to exclude lymphoma, a bone marrow biopsy and a fine needle biopsy of the cervical lymph nodes were performed. The lymph node biopsy revealed a few activated large lymphocytes that suggested reactive lymphoid hyperplasia, but EBV in situ hybridization was negative. The bone marrow was normocellular, showing reactive lymphocytosis, and the PCR result for EBV DNA in the bone marrow showed a viral load of 2,982 copies/mL despite the previous negative result for EBV on in situ hybridization. After admission, on the impression of lymphoma or infectious mononucleosis, we administered adequate hydration by intravenous method. We initiated treatment with prednisolone (0.5 mg/kg/day) on the third hospital day and acyclovir (30 mg/kg/day) on the fourth hospital day, on the impression of infectious mononucleosis, because there was a sustained fever and liver enzyme abnormalities. After this treatment, on the fourth hospital day, the liver enzyme levels decreased and the fever subsided. Also, our patients no longer complained of abdominal pain. Follow-up abdominal ultrasonography on the fifth hospital day showed diffuse hepatosplenomegaly, no sonographic evidence of splenic infarction or other focal lesion of the spleen, and ascites with diffuse gallbladder wall edema (Fig. 1C). The liver enzyme levels on the eighth hospital day were revealed AST, 38 IU/L; ALT, 58 IU/L; ALP, 138 IU/L; γ-GTP, 310 IU/L; LDH, 427 IU/L. After 10 days of conservative treatment, the patient was discharged. Two months after discharge, all liver function test results were normal and PCR in peripheral blood for EBV DNA showed a viral load of 220 copies/mL. Five months after discharge, the serum EBV-VCA IgG analysis was positive and follow up EGD revealed linear red streak detected on the body large curvature side (Fig. 2C, D).
EBV-associated diseases are infectious mononucleosis; various organs can be affected by these diseases, acute complications may occur due to infectious mononucleosis and even fulminant infection such as hemophagocytic lymphohistiocytosis may be occurred [5]. The EBV can affect any organic system and has been associated with diverse disease manifestations such as pneumonia, myocarditis, pancreatitis, mesenteric adenitis, myositis, glomerulonephritis, and splenic infarction. In addition, primary EBV infection has been associated with a large number of neurologic syndromes such as Guillain-Barré syndrome, facial nerve palsy, meningoencephalitis, aseptic meningitis, transverse myelitis, peripheral neuritis, and optic neuritis [6]; and hematologic abnormalities such as hemolytic anemia, thrombocytopenia, aplastic anemia, thrombotic thrombocytopenic purpura, and hemolytic-uremic syndrome [7].
The serological tests for EBV and extraction of the heterophile antibody can be used for the diagnosis of EBV infection. Serum specific antibodies for EBV detection are viral capsid antigen IgM/IgG (VCA IgM/IgG), EBV early antigen IgG/IgM (EA IgG/IgM), and Epstein-Barr nuclear antigen (EBNA) IgG. In addition, the PCR quantification for EBV DNA can be used. Our patient presented with the fever, fatigue, and generalized lymphadenopathy, and PCR for EBV DNA in blood and bone marrow aspiration, also EBV in situ hybridization in gastric mucosa confirmed the diagnosis of EBV infectious mononucleosis.
Because infectious mononucleosis is a self-limiting disease, treatment of choice of infectious mononucleosis is conservative treatment. Patients are encouraged to take adequate rest and if they have fever or sore throat, acetaminophen or nonsteroidal anti-inflammatory drugs are recommended. The use of corticosteroids or anti-viral agent such as acyclovir in the treatment of infectious mononucleosis has been controversial. Short courses of corticosteroids can be helpful for some complication of infectious mononucleosis such as airway obstruction, hemolytic anemia, seizures, and meningitis [8]. Tynell et al. [9] described the combination of acyclovir and prednisolone reduced oropharyngeal shedding of the virus but did not affect the duration of symptoms or lead to an earlier return to school or work. In our study, acyclovir and prednisolone were used to reduce oropharyngeal shedding of virus because the patient was in the third grade of high school. In addition, patient had severe complications of infectious mononucleosis with gastritis and splenic infarction accompanied by severe abdominal pain. However, because these treatments are not sufficient to recommend for symptom relief, the use of acyclovir and corticosteroid should be carefully determined.
The gastrointestinal manifestations associated with EBV are diverse and range from acute gastritis to malignancy. Acute gastritis and chronic gastritis can be seen in EBV infection cases and it is difficult to distinguish it from other types of gastritis such as Helicobacter gastritis and lymphocytic gastritis [10]. The endoscopic findings of EBV gastritis are various, ranging from mucosal hypertrophy with partial edema to ulcerative lesions with irregular margins [1112].
The gastrointestinal cancers associated with EBV are non-Hodgkin lymphoma, gastric T-cell lymphoma, gastric adenocarcinoma, esophageal cancer, and rectal cancer [131415]. Most of the gastric lymphoma cases are non-Hodgkin's lymphoma with a B-cell origin, and T-cell lymphoma is rare. Jung et al. [13] described that the upper endoscopic findings of EBV-associated primary gastric T-cell lymphoma are related to a deep ulcerous lesion with blood vessels associated with gastrointestinal bleeding.
As there are no specific endoscopic findings for the diagnosis of EBV infection, performing biopsies with in situ hybridization could be helpful to confirm the diagnosis. EGD in our patient showed generalized hyperemic nodular lesions in the stomach. The patient could be diagnosed on the basis of the findings on biopsies with in situ hybridization and the pain was relieved with a proton pump inhibitor.
Splenic infarction is an uncommon complication but it occurs in various diseases such as hematologic disease, cardioembolic disease, diseases with a hypercoagulable state, septic emboli, splenic vascular disease, and collagen vascular disease [1617]. The splenic infarction is an extremely rare thromboembolic complication of the acute EBV infection especially in a previously healthy individual. In a 10-year retrospective study in adults with splenic infarction, only 3 were identified with positive EBV serology [17]. Pathophysiologic mechanism of splenic infarction has not yet been elucidated in acute EBV infection, but the association between infection and anti-phospholipid antibodies has been raised in several studies [18]. In cases of splenic infarction due to infectious mononucleosis complication, hypercoagulability caused by transient protein C and protein S deficiency or transient anti-phospholipid antibodies is thought to be the cause. Therefore, laboratory tests such as anti-phospholipid antibody, protein S activity, protein C activity, protein C activity, lupus anticoagulant, and antinuclear antibodies are recommended. In our case, only PT and aPTT were done which were normal and splenic infarction improved after 5 days of intravenous hydration and supportive care under admission.
The most common symptom of splenic infarction is left upper quadrant pain, but as this symptom can be presented in several diseases, it is difficult to detect splenic infarction as a cause of abdominal pain. Among patients with EBV infectious mononucleosis, splenic infarction is a very rare complication; therefore, splenic infarction is likely to be overlooked in these clinical scenarios. Splenic infarction can be diagnosed by using radiologic imaging techniques such as abdominal ultrasonography, CT, or magnetic resonance imaging. The most common CT finding of splenic infarction was an area of decreased attenuation within the splenic parenchyma and ultrasonographic feature is usually that of a hypoechoic or an anechoic area in the spleen [19]. If a patient diagnosed with infectious mononucleosis complains of abdominal pain, an imaging study should be performed keeping in mind a possible spleen infarction.
Treatment choice of splenic infarction is supportive care which consists of rest, hydration, and analgesics [15]. In most cases, this disease is self-limiting with resolution of symptoms in 7 to 14 days. However, if severe complications such as splenic rupture occur, surgical treatment such as splenectomy may be necessary. Radiologic follow-up of splenic infarction has not been established yet, but complications such as splenic rupture are most likely to occur between 4 and 21 days after infectious mononucleosis, so follow-up is required regularly during this period [20]. Our patient underwent abdominal ultrasonography on the fifth day of admission, and splenic infarction was improved. After that, abdominal ultrasonography was performed again at 5 months after discharge. No focal lesion was found in spleen. This suggests that the patient's recovery from splenic infarction is faster than in the previous studies, and it was most probably due to early detection and administration of supportive care.
In conclusion, we reported a case of EBV-related gastritis and splenic infarction in a previous healthy 16-year-girl presenting with fever, fatigue, and epigastric pain. This is the first case report of both spleen infarction and EBV-related gastritis, a rare complication in infectious mononucleosis. In EBV infection cases, owing to various clinical features the infection, it is important to closely monitor patients with infectious mononucleosis and to diagnose and treat complications by using appropriate tools and therapeutic regimens.
Figures and Tables
Fig. 1
Abdominal and pelvis computed tomography (CT) showed (A) hepatosplenomegaly and 2×1.6 cm, sized low attenuated lesion (white arrow), which that was consistent with splenic infarction; and (B) multiple slightly enlarged lymph nodes around aorta and mesentery (white arrows). Follow-up abdominal ultrasonography on the fifth hospital day showed (C) no sonographic evidence of splenic infarction or other focal lesion of the spleen.
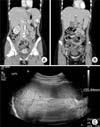
Fig. 2
Characteristics of the patient's esophagogastroduodenoscopy (EGD) image Endoscopic image showing (A) hyperemic nodular lesion (asterisk) and (B) linear erosions (arrows). Five months after discharge, follow up EGD revealed (C, D) linear red streak detected on the body large curvature side (arrows).
References
1. Luzuriaga K, Sullivan JL. Epstein-Barr virus. In : Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. 3rd ed. Washington, DC: ASM Press;2009. p. 521–536.
2. Evans AS, Niederman JC, Cenabre LC, West B, Richards VA. A prospective evaluation of heterophile and Epstein-Barr virus-specific IgM antibody tests in clinical and subclinical infectious mononucleosis: specificity and sensitivity of the tests and persistence of antibody. J Infect Dis. 1975; 132:546–554.

3. Kim JM, Song CW, Song KS, Kim JY. Acute gastritis associated with Epstein-Barr virus infection in a child. Korean J Pediatr. 2016; 59:Suppl 1. S68–S71.

4. Gang MH, Kim JY. Splenic infarction in a child with primary Epstein-Barr virus infection. Pediatr Int. 2013; 55:e126–e128.

6. Tselis A, Duman R, Storch GA, Lisak RP. Epstein-Barr virus encephalomyelitis diagnosed by polymerase chain reaction: detection of the genome in the CSF. Neurology. 1997; 48:1351–1355.

7. Haller A, von Segesser L, Baumann PC, Krause M. Severe respiratory insufficiency complicating Epstein-Barr virus infection: case report and review. Clin Infect Dis. 1995; 21:206–209.

8. Jenson HB. Epstein-Barr virus. In : Kliegman R, Stanton B, St. Geme J, Schor N, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Elsevier;2015. p. 1586–1590.
9. Tynell E, Aurelius E, Brandell A, Julander I, Wood M, Yao QY, et al. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double-blind, placebo-controlled study. J Infect Dis. 1996; 174:324–331.

10. Hisamatsu A, Nagai T, Okawara H, Nakashima H, Tasaki T, Nakagawa Y, et al. Gastritis associated with Epstein-Barr virus infection. Intern Med. 2010; 49:2101–2105.

11. Zhang Y, Molot R. Severe gastritis secondary to Epstein-Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med. 2003; 127:478–480.
12. Kitayama Y, Honda S, Sugimura H. Epstein-Barr virus-related gastric pseudolymphoma in infectious mononucleosis. Gastrointest Endosc. 2000; 52:290–291.

13. Jung JK, Chung JP, Kim KC, Park HJ, Lee KS, Chon CY, et al. A case of Epstein-Barr virus-associated primary gastric T-cell lymphoma with rapidly progressive endoscopic features and clinical course. Korean J Gastrointest Endosc. 1999; 19:438–442.
14. Ueo T, Kashima K, Daa T, Kondo Y, Yokoyama S. Coexistence of Epstein-Barr virus-associated gastric carcinoma with malignant lymphoma: report of two cases. Virchows Arch. 2006; 449:215–219.

15. Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004; 350:1328–1337.

16. Jaroch MT, Broughan TA, Hermann RE. The natural history of splenic infarction. Surgery. 1986; 100:743–750.
17. Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med. 2009; 27:262–265.

18. García-Carrasco M, Galarza-Maldonado C, Mendoza-Pinto C, Escarcega RO, Cervera R. Infections and the antiphospholipid syndrome. Clin Rev Allergy Immunol. 2009; 36:104–108.

19. Maier W. Computed tomography in the diagnosis of splenic infarction. Eur J Radiol. 1982; 2:202–204.
20. Asgari MM, Begos DG. Spontaneous splenic rupture in infectious mononucleosis: a review. Yale J Biol Med. 1997; 70:175–182.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download