Abstract
Bannayan-Riley-Ruvalcaba syndrome (BRRS) is one of the phosphatase and tensin homolog hamartoma tumor syndrome with a PTEN gene mutation. It is a rare dominant autosomal disorder characterized by cutaneous lipomas, macrocephaly, intestinal polyps, and developmental delay. Diagnosing this syndrome is important, because it may represent the pediatric phenotype of Cowden syndrome, in which there is an increased risk for malignant tumors in children. Until now, the prevalence of BRRS is unknown. Several dozen cases have been reported in the medical literature, but no case has been reported in Korea. Here we report a case of a 19-year-old girl who was diagnosed with BRRS because of macrocephaly, intellectual disability, and intestinal polyps. Her mother had similar findings and a PTEN mutation. Neither patient had mutations detected by conventional mutation-detection techniques, but a PTEN gene deletion was demonstrated by chromosomal microarray analysis.
Bannayan-Riley-Ruvalcaba syndrome (BRRS) is one of the phosphatase and tensin homolog hamartoma tumor syndromes (PHTS). It is a rare dominant autosomal disorder characterized by cutaneous lipomas, macrocephaly, intestinal polyps, and developmental delay associated with PTEN gene mutation (tumor suppressor gene deletion on chromosome 10q22-q23) [1]. This disease encompasses three previously described disorders, such as Bannayan-Zonana syndrome, Riley-Smith syndrome, and Ruvalcava-Myhre-Smith syndrome [2]. In 1971, Bannayan [3] reported the congenital combination of macrocephaly with multiple subcutaneous and visceral lipomas and hamangiomas. Then, in 1980, Ruvalcaba described two males with macrocephaly, harmatomatous intestinal polyposis, and pigmentary spotting of the penis [2]. The prevalence of BRRS is unknown but several dozen cases have been reported in the medical literature. Researchers suspect that the disorder is under-diagnosed because its signs and symptoms vary and some are subtle [45].
Although there are no international consensus criteria for diagnosing BRRS, several groups of investigators have proposed criteria to facilitate clinical diagnosis [6]. Marsh et al. [7] defined a clinical diagnosis of BRRS as meeting three out of four features: macrocephaly, lipomatosis, hemangiomas and speckled pigmented maculae on the penis. Parisi et al. [8] defined the syndrome in patients with two of three of the following features of macrocephaly, hamartomas (including at least one lipoma, hemangioma, or intestinal polyp), and penile macules in males.
A chromosomal analysis for PTEN gene mutations also serves as a useful diagnostic tool.
The prevalence of identified germline PTEN mutations in PHTS varies widely, with Cowden syndrome (CS) having 80% prevalence of identified intragenic PTEN mutations, BRRS with 65% prevalence, and Proteus syndrome with <20% prevalence [6].
Here, we report a case of a 19-year-old girl who was diagnosed with BRRS because of macrocephaly, intellectual disability and intestinal polyps as well as PTEN gene mutation.
A 19-year-old female patient was admitted to Gachon University Gil Medical Center (Incheon, Korea) because of a history of refractory iron deficiency anemia (IDA) and recurrent gastroenteritis.
She first visited our outpatient clinic because of recurrent gastroenteritis 4 years prior. Laboratory investigation was normal except for IDA. She was put on iron tablets and regular follow-up at our outpatient department, but her anemia did not improve. She denied any history of skipped medicine. So, we recommended the endoscopic study 3 years ago because of the suspicion of a gastrointestinal bleeding or hamartomatous polyposis syndrome, but she refused at that time; however, 3 years later it could be performed.
The patient had a history of mild mental retardation at the age of 8 years as her mother. Their intelligence quotient score ranged from 50-75. She had one sister who was reported as normal.
General physical examination revealed moderate pallor, normal oral cavity. There was no jaundice, cyanosis, edema, thyromegaly, lymphadenopathy, clubbing, or scoliosis. There were no dermatologic abnormalities or any abnormality affecting the genitalia. Anthropometric examination revealed macrocephaly with head circumference of 608 mm (for reference, the average and 99th percentile occipito-frontal circumferences of 19- and 24-year-old Korean females are 552 and 585 mm, respectively), height 162.8 cm (55-75th percentile), weight 62 kg (90-95th percentile). Her sister and mother showed no macrocephaly.
Lab investigations revealed hypochromic microcytic anemia with hemoglobin 8.4 g/dL and mean cell volume 68.8, ferritin 24.8 µg/L, iron/total iron binding capacity ratio 6.6% and stool occult blood testing was negative.
Gastrofibroscopy and colonoscopy revealed multiple polyps of different sizes and shapes in the terminal ileum, stomach, and duodenum (Fig. 1). Several biopsies confirmed inflammatory and hyperplastic polyps.
In family screening, her sister and mother were called and examined. Her sister was found as normal but her mother revealed multiple polyps on stomach, and duodenum.
The patient also had an intellectual disability and the physical examination revealed a head circumference suggestive of macrocephaly.
Considering all symptoms and the results of the examinations, BRRS was the most likely clinical diagnosis. Although imaging studies including brain magnetic resonance imaging were required, they were not conducted because of cost.
PTEN gene mutation analysis was conducted using DNA extracted from peripheral blood leukocytes. No mutations were detected by conventional polymerase chain reaction (PCR) mutation-deletion techniques or direct sequencing with reference to the NM_000314.4 m-RNA sequence. Thus, we conducted a chromosomal microarray analysis (CMA) using the Affymetrix Cytoscan 750k array with reference to the human gene ver. 19 (Affymetrix, Santa Clara, CA, USA). As a result, an approximates 240 kb microdeletion was detected in the 10q23.31 region (Fig. 2).
An approximate 220 kb microdeletion was detected in her mother in the 10q23.31 region. Her older sister had no such findings.
BRRS is associated with germline mutations of the PTEN tumor suppressor gene, which has a significant role in cellular proliferation, migration, and apoptosis pathways. PTEN mutations are seen in up to 65% of patients with a suspected PHTS diagnosis [910].
Three other clinically distinct syndromes are associated with PTEN mutations and are collectively referred to as PHTS. These allelic disorders include CS, Proteus syndrome, and Proteus-like syndrome, and all have established diagnostic criteria. However, no BRRS diagnostic criteria have been established [211].
BRRS can be diagnosed based on clinical observations, including the presence of macrocephaly, lipomas, hamartomatous intestinal polyposis, developmental delay and mental retardation, as well as pigmented macules on the glans penis in males.
At least half of affected patients have macrocephaly, and many also have a high birth weight. Growth usually slows during childhood, so affected adults are of normal height and body size. Gontijo et al. [12] reported BRRS with deforming lipomatous hamartomas in infant. Intestinal polyps mostly present in childhood through chronic anemia, diarrhea or invagination of the small bowel. The signs and symptoms of BRRS are present from birth or become apparent in early childhood. Our patient's past medical history was significant for recurrent gastroenteritis from childhood and refractory IDA.
BRRS and CS reportedly share clinical characteristics and represent a single entity. However, almost everyone with CS develops hamartomas. These growths are most commonly found on the skin and mucous membranes (such as the lining of the mouth and nose), but they can also occur in the intestine and other parts of the body. The growth of hamartomas on the skin and mucous membranes typically becomes apparent by a person's late twenties [45].
No such criteria exist for BRRS, but the syndrome is suspected in the presence of macrocephaly, hamartomatous intestinal polyposis, and mental retardation, all of which were present in our patient. At present, she most closely fits BRRS, although further development of symptoms with time may eventually lead to the diagnosis of CS.
Although an increased risk for malignancies in patients with BRRS has not been documented, some authors recommend that patients with BRRS comply with the same malignancy screening recommendations for patients with CS because of the clinical and genetic similarities between CS and BRRS and because patients with PTEN mutations have increased risk for cancers [78]. Therefore, early diagnosis is important.
Genetic testing is available to identify mutations and/or deletions within PTEN. Identification of such alterations provides confirmation of the PHTS diagnosis and further permits predictive testing and prenatal diagnosis within affected families [11]. Several methodologies are currently used to detect PTEN mutations [13].
The appropriate order of PTEN testing to optimize yield first includes sequencing all PTEN coding exons 1-9 and flanking intronic regions. If no pathogenic variant is identified, deletion/duplication analysis is recommended [14]. So, we conducted conventional PCR mutation-deletion techniques and direct sequencing. Since no mutation was identified, we performed deletion analysis by microarray. Previous study showed that the detection rates of microarray ranged from 5% to 17% for children undergoing a genetics evaluation for a variety of conditions who previously had a karyotype with no chromosome abnormalities [15]. Menko et al. [16] reported variable phenotypes associated with 10q23 microdeletions detected by microarray.
Recently, some consensus statements have proposed utilization of CMA as a first-line test in patients with multiple congenital anomalies not specific to a well delineated genetic syndrome, developmental delay and intellectual disability, or autism spectrum disorders. CMA enables genome-wide detection of submicroscopic chromosomal abnormalities with greater precision and accuracy [17]. Although CMA has distinct advantages, there are several limitations, including its inability to detect balanced chromosomal rearrangements and low-level mosaicism, its interpretation of copy number variants of uncertain clinical significance, and significantly higher costs. Thus, CMA is not currently a replacement for conventional cytogenetics but can be used as an adjunct to conventional cytogenetics to identify chromosomal abnormalities, leading to a more accurate and comprehensive assessment of chromosomal aberrations [1819].
In conclusion, our case study suggests that CMA can be used to identify chromosomal abnormalities even if conventional cytogenetics testing was normal in BRRS.
Figures and Tables
Fig. 1
(A) Colonoscopy showed multiple polyps on the terminal ileum. Some flat polyps without neck were noted on the rectum. (B) Gastrofibroscopy showed small multiple polyps on the stomach and duodenum.
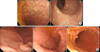
Fig. 2
(A) Karyogram of the patient. The deletion in the long arm of chromosome 10 (arrow). Left: the patient, right: her mother. (B) Chromosomal microarray profile of chromosome 10. The X-axis represents the probe index on chromosome 10, and the Y-axis represents the signal log2 ratio of the probe. The 10q23.31 region showed a 220kb micro-deletion in Chr10. Left: the patient, right: her mother.

References
1. Buisson P, Leclair MD, Jacquemont S, Podevin G, Camby C, David A, et al. Cutaneous lipoma in children: 5 cases with Bannayan-Riley-Ruvalcaba syndrome. J Pediatr Surg. 2006; 41:1601–1603.

2. Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005; 100:476–490.

3. Bannayan GA. Lipomatosis, angiomatosis, and macrencephalia. A previously undescribed congenital syndrome. Arch Pathol. 1971; 92:1–5.
4. Genetics Home Reference. Bannayan-Riley-Ruvalcaba syndrome [Internet]. Bethesda: U. S. National Library of Medicine;2012. cited 2016 Mar 13. Available from: https://ghr.nlm.nih.gov/condition/bannayan-riley-ruvalcaba-syndrome.
5. Genetics Home Reference. Cowden syndrome [Internet]. Bethesda: U. S. National Library of Medicine;2012. cited 2016 Mar 13. Available from: https://ghr.nlm.nih.gov/condition/cowden-syndrome.
7. Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998; 7:507–515.

8. Parisi MA, Dinulos MB, Leppig KA, Sybert VP, Eng C, Hudgins L. The spectrum and evolution of phenotypic findings in PTEN mutation positive cases of Bannayan-Riley-Ruvalcaba syndrome. J Med Genet. 2001; 38:52–58.

9. Latiff ZA, Atmawidjaja RW, RajaLope RJ, Syed Omar SA, Syed Zakaria SZ, Jamal RA. Bannayan Riley Ruvalcaba syndrome. Ann Acad Med Singap. 2010; 39:578–582.
10. Lachlan KL, Lucassen AM, Bunyan D, Temple IK. Cowden syndrome and Bannayan Riley Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: results of a clinical study of PTEN mutation carriers. J Med Genet. 2007; 44:579–585.

12. Gontijo GM, Pinto CA, Rogatto SR, Cunha IW, Aguiar S Jr, Alves CA. Bannayan-Riley-Ruvalcaba syndrome with deforming lipomatous hamartomas in infant--case report. An Bras Dermatol. 2013; 88:982–985.

13. Zhou XP, Waite KA, Pilarski R, Hampel H, Fernandez MJ, Bos C, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet. 2003; 73:404–411.

14. Eng C. PTEN hamartoma tumor syndrome (PHTS) [Internet]. Pagon RA, Adam MP, Ardinger HH, editors. GeneReviews. Seattle: University of Washington;2001. 11. 29. 2014 Jan 23. 2016 Mar 13. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1488/.
15. Shaffer LG, Bejjani BA. Using microarray-based molecular cytogenetic methods to identify chromosome abnormalities. Pediatr Ann. 2009; 38:440–447.

16. Menko FH, Kneepkens CM, de Leeuw N, Peeters EA, Van Maldergem L, Kamsteeg EJ, et al. Variable phenotypes associated with 10q23 microdeletions involving the PTEN and BMPR1A genes. Clin Genet. 2008; 74:145–154.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download