Abstract
Purpose
Recently, vitamin D insufficiency has increased and has been correlated to growth and puberty in children. This study was conducted to find the prevalence of subclinical vitamin D insufficiency and its influence on school-aged children in Korea.
Methods
The subjects of this study were 397 children aged 7 to 15 years who had been tested for 25-OH vitamin D3 among the outpatients of the Department of Pediatrics in Eulji General Hospital from March 2007 to February 2011. Data for age, sex, comorbidities, serum 25-OH vitamin D3, height, weight, body mass index (BMI), and sunlight exposure time were collected before and after 3 months of vitamin D administration, retrospectively.
Results
Vitamin D insufficiency was present in 343 (86%) of the subjects. In the vitamin D insufficient group, chronological age was 8.96±1.72 years, mean height (z-score [z]) was 0.51±1.26, mean BMI (z) was 0.81±2.20, and bone age was 10.26±1.75 years. In the vitamin D sufficient group, chronological age was 9.61±1.77 years, mean height (z) was-0.66±0.98, mean BMI (z) was-0.01±1.16, and bone age was 9.44±2.12 years. A paired t-test showed that three months after vitamin D administration, the mean 25-OH vitamin D3 level in the insufficient group increased to 24.38 ±10.03 ng/mL and mean BMI (z) decreased to 0.67±1.06.
Vitamin D plays an important role in the growth and development of infants. It is essential for accelerating calcium absorption in the intestine and therefore has a vital role in the mineralization of bone [1].
As nutrition has improved, rickets caused by vitamin D deficiency was thought to have almost disappeared. However, many recent studies have reported an increase in vitamin D deficiency worldwide [2]. Moreover, as the incidence of rickets, which had initially been decreasing, has recently been increasing, vitamin D deficiency due to an increase of breast-feeding has been highlighted [3,4] and vitamin D insufficiency is expected to be prevalent among school-aged children [5]. Furthermore, many studies have reported that even normal healthy persons who have no clinical symptoms can have vitamin D deficiency [6,7]. In addition, recent evidence suggests that obesity itself is an independent risk factor for vitamin D insufficiency and that body fat is inversely proportional to the level of 25-OH vitamin D3 in the serum [8]. Although the criteria for vitamin D deficiency are controversial, it has been reported that parathyroid hormone levels increase to compensate for the deficiency from 30 ng/mL or less of 25-OH vitamin D3 in general so that it has been recommended for healthy persons that the serum levels of 30 ng/mL or more of 25-OH vitamin D3 should be maintained. Therefore, many studies have used 30 ng/mL of 25-OH vitamin D3 in serum as a criterion to distinguish between normal and shortage groups with the shortage group subdivided into insufficient and deficient groups, however this is controversial [5,9]. In Korea, an increasing prevalence of vitamin D deficiency has been reported as well; however, most studies have concentrated on the correlation between the prevalence of vitamin D deficiency and the growth of infants aged less than 2 years old [10,11]. In contrast, a few studies [12,13] have been done on the effects of vitamin D insufficiency in school-aged children and adolescent in Korea.
Thus, this study was conducted to find out the prevalence and effects of subclinical vitamin D insufficiency in school-aged children in Korea, as well as the way to replenish vitamin D insufficiency.
The subjects of this study comprised 397 children aged between 7 and 15 years, who were tested for 25-OH vitamine D3 among the outpatients of the Department of Pediatrics in Eulji General Hospital from March 2007 to February 2011 for growth assessment. Subjects who had underlying diseases that could affect growth (skeletal dysplasia, chromosomal disorders, growth hormone deficiency, thyroid hormone dysfunction, gonadal disorders, and chronic systemic disease) were excluded from the study.
The survey was conducted with 397 children together with their parents, data for ages, sex, comorbidities, levels of 25-OH vitamine D3, height, weight, body mass index (BMI), growth velocity and sunlight exposure time (hour/week) were collected. This data was compared with the data for height, weight, BMI, levels of 25-OH vitamine D3 and change in growth velocity after oral administration of vitamin D for a follow-up period of 2.7-4.6 months (mean: 3.5 months) retrospectively. Height was measured with a Harpenden Stadiometer (Holtain Ltd., Crosswell, UK) while weight was measured with an electronic scale (GL-6000; G-TECH International Co., Ltd., Uijeongbu, Korea). Bone age was measured using the Greulich-Pyle method after taking a plain radiograph of the left hand and wrist. The z-scores of height, weight and BMI were calculated using the growth curve of the children and adolescents in Korea in 2007. We assessed sunlight exposure time (hour/week) by asking the parents how many hours of various outdoor activities were performed by the subjects [14]. Levels of 25-OH vitamine D3 were measured using chemiluminiscent immunoassay. In this study, a serum level of 25-OH vitamin D3 less than 30 ng/mL was defined as vitamin D insufficiency and more than 30 ng/mL was defined as vitamin D sufficiency.
Oral cholecalciferol 600 IU/day was administered to each insufficient subject according to the Institute of Medicine dietary guidelines [15].
The study design was approved by the institutional review board of the Eulji Hospital Medical Center (2013-07-022). Informed consent was waived by the board because of retrospective review of medical records.
For statistical analysis, SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used and the results were represented as mean value±standard deviation. Comparison of the mean values between the vitamin D insufficient group and the vitamin D sufficient group was done with a parametric test using the Student's t-test while the comparison of the differences in data before and after oral vitamin D administration was done with a paired t-test. For all the tests, a p<0.05 was considered to indicate statistical significance.
In total, 397 children, including 105 boys and 292 girls (26.4% and 73.6% of the total number of children examined, respectively), were examined. The mean chronological age was 9.04±1.75 years and the mean bone age was 10.15±1.83 years. The mean value of 25-OH vitamine D3 measured prior to vitamin D administration was 21.22±14.09 ng/mL, which was below the value used to define vitamin D sufficiency (30 ng/mL), and the mean weight and height were 33.24±7.93 kg [mean weight (z-score; z), 0.64±1.53] and 134.83±9.04 cm [mean height (z), 0.35±1.29], respectively. In addition, BMI was 18.08±2.73 kg/m2 [BMI (z), 0.69±2.11] and the mean growth velocity was 6.40±2.54 cm/year. The mean sunlight exposure time of the children was 3.42±2.41 hr/week (Table 1).
The mean value for 25-OH vitamin D3 was only above 30 ng/mL for the 12 year age group, for which it was 30.35±16.53 ng/mL. For all the other age group, the mean value for 25-OH vitamine D3 was <30 ng/mL, indicating that approximately 86% of the children examined had vitamin D insufficiency (Fig. 1).
At the beginning of the treatment, there were more children in the vitamin D insufficient group (343 [86%]) than in the sufficient group (54 [14%]). In particular, there were more girls than boys in the insufficient group. The chronological age in the vitamin D insufficient group was 8.96±1.72 years, which was less than that in the vitamin D sufficient group (9.61±1.77 years), while the bone age in the vitamin D insufficient group was 10.26±1.75 years, which was more than that in the vitamin D sufficient group (9.44±2.12 years; p<0.05). The difference between bone age and chronological age was higher in the vitamin D insufficient group.
The mean weight (z) for the vitamin D insufficient group was 0.80±1.53, which was greater than the value for the sufficient group (-0.38±1.12). The mean height (z) in the vitamin D insufficient group was 0.51±1.26, which was also higher than the value for the sufficient group (-0.66±0.98; p<0.05). The mean growth velocity in the vitamin D insufficient group was 6.52±2.59 cm/year, which was greater than the value for the sufficient group (5.03±1.25 cm/year), although no significant difference was shown between them. The mean BMI (z) for the insufficient group was 0.81±2.20, which was higher than the value for the sufficient group (-0.01±1.16; p<0.05) (Table 1).
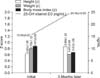
After mean 3.5 months of treatment with vitamin D, the mean 25-OH vitamin D3 level in the serum of the subjects increased significantly from 15.54±5.87 ng/mL to 24.38±10.03 ng/mL. In addition the average height (z) increased significantly from 0.63±1.26 to 0.86±1.23 after vitamin D administration (p<0.05). On the other hand, the mean weight (z) decreased from 0.79±1.12 to 0.61±3.89, together with the mean BMI (z), from 0.87±2.82 to 0.67±1.06, although these differences were not significant (Fig. 2).
In this study, we showed that, in Seoul, Korea, 86% of the school-aged children examined had vitamin D insufficiency. In addition, we showed that vitamin D insufficiency had a tendency to increase BMI and advance bone age. And vitamin D insufficiency remained even though vitamin D was administered according to the guideline.
Several previous studies have reported similar results as our study. Neyestani et al. [16] reported that 86% of children in Tehran had vitamin D insufficiency, while their result was based on the definition of vitamin D insufficiency as less than 37.0 nmol/L (15 ng/mL) of 25-OH vitamine D3. Weng et al. [17] also reported that about 68% of children examined in Philadelphia, Pennsylvania during the winter time were vitamin D insufficient (serum levels of 25-OH vitamine D3<30 ng/mL). Thus, although the criteria were different from region to region, 70% to 90% of school age children have been reported to be vitamin D insufficient.
It has been known that vitamin D insufficiency is associated with dark skin pigmentation or race, female gender, living in a northern latitude, lack of direct sun exposure, and the winter season [18]. Therefore, we suspect that the reasons for the extent of vitamin D insufficiency seen in this study could be latitude, indoor urban lifestyle, and short sun exposure time. In countries that are in a similar latitude as South Korea (33°-43°), such as Italy (38°-45°), Spain (37°-43°), and the USA (25°-47°), serum levels of 25-OH vitamine D3 were all within the range of vitamin D insufficiency (22.36 ng/mL in Italy, 23.96 ng/mL in Spain, and 27.36 ng/mL in the USA [19]). In addition, an indoor urban lifestyle and short sun exposure time might also affect vitamin D insufficiency, as reported in the study of Choi et al. [20]. Additionally, they reported that vitamin D insufficiency (<30 ng/mL 25-OH vitamin D3) was found in 86.7% of males and 93.4% of females in all groups in Korea. In particular, they reported that the mean 25-OH vitamin D3 value for girls was lower than that of boys in the puberty age group (10 to 19 years). In our study, the mean serum 25-OH vitamine D3 value in the insufficient group was 17.00±6.03 ng/mL, and the ratio of girls to boys in the insufficient group was 3 : 1, as opposed to 1 : 1 in the sufficient group, which showed that there were more girls in the insufficient group. We initially hypothesized that the reason for this result might be that the girls had shorter sun exposure time and higher BMI (z) than the boys, but there was no significant difference in either the sun exposure time or the BMI (z) values between boys and girls in the insufficient group in 3-month follow-up period. Therefore, long term follow up may be required.
Chronological age in the insufficient group was less than that in the sufficient group but the bone age was greater and growth velocity was faster in the insufficient group. Similarly, in 2001, Villamor et al. [21] reported that school-age girls with insufficient vitamin D underwent menarche about one year earlier than children with sufficient vitamin D. Even though the authors could not explain direct causality, they suggested the mechanisms underlying their observations might be quick progress to obesity or an increase in insulinlike growth factor 1. Likewise, we hypothesize that growth in the insufficient group might conclude sooner than in the sufficient group, so more long term follow up studies should be conducted. In the present study, the insufficient group had higher values for mean weight (z), mean height (z), and mean BMI (z) than the sufficient group, which is in contrast to the results from a previous study showing lower growth in the vitamin D insufficient group [22]. We observed a higher mean BMI (z) in the insufficient group. It is known that obesity-associated vitamin D insufficiency is likely to be because of a decreased bioavailability of vitamin D3 from cutaneous and dietary sources, as vitamin D3 is deposited in body fat compartments [23].
In our study, the mean value for 25-OH vitamine D3 for the 12 years age group is above 30 ng/mL (30.35 ng/mL; Fig. 2). However, that group comprised only 15 (4%) people. The analysis from this retrospective study suggests that the investigated data could have had such results due to the small number of subjects in the 12 years age group.
After mean 3.5 months of vitamin D administration, the serum level of 25-OH vitamin D3 increased, as did mean height (z), whereas mean weight (z) and BMI (z) decreased. But the decrease in BMI (z) was not significant.
In this study, we administered 600 IU/day to the subclinical vitamin D insufficient subjects according to Institute of Medicine dietary guidelines, although there have been no absolute guideline about vitamin D supplementation to treat vitamin D insufficiency. Misra et al. [24] recommend that all infants and children who do not ingest at least 1 L of vitamin D-fortified milk per day, receive vitamin D 400 IU/day as a supplement. And for children aged 1-18 years who are vitamin D insufficient, a treatment regimen consisting of 2,000 IU/day of vitamin D2 or vitamin D3 for at least 6 weeks or with 50,000 IU of vitamin D2 once a week for at least 6 weeks to achieve a blood level of 25-OH vitamin D3 above 30 ng/mL, followed by maintenance therapy of 600-1,000 IU/day was suggested by Holick et al. [25]. Because the vitamin D level was increased after 3 months of vitamin D administration, but was still less than 30 ng/mL, vitamin D supplementation greater than what we administered in this study may be needed. The latter suggestion is considered more appropriate to our study.
A negative correlation between BMI (z) and serum vitamin D concentration has been proposed in many studies [26,27]. The present study showed a negative correlation between BMI (z) and changes in 25-OH vitamine D3 serum levels, comparing the results before and after vitamin D administration (Fig. 2). Viljakainen et al. [28] claimed that acceleration of bone mineral accretion was found in the femur and lumbar spine in adolescent girls, after they took oral vitamin D, showing decreased bone resorption and increased bone mineral content, which could account for the increase in mean height (z) and the mean growth range after taking oral vitamin shown in the present study.
However, in this study, because we administered vitamin D only to those patients diagnosed with vitamin D insufficiency, there was no follow up data for the control group. In addition, there was only mean 3.5 months of vitamin D administration and there were differences in the starting ages for oral vitamin D administration. These were the limitations of our study. Factors such as inconsistent seasons, latitude and race were not considered during the follow up in the present study. Additional studies will be required as these considerations should be taken into account. And sun exposure time, which has been proposed to be the most influential factor on serum levels of 25-OH vitamin D3 in previous studies [29] did not appear to affect the levels of 25-OH vitamin D3.
In spite of these limitations, this study is important because it focused on school-aged children, in contrast to previous studies which were mainly about infants. Also, we suggest that doses of oral cholecalciferol greater than 600 IU/day may be needed for vitamin insufficient groups because even though we administered oral cholecalciferol at 600 IU/day for three months, the serum 25-OH vitamin D3 level remained <30 ng/mL; We suspect this, as well as the short time spent by the children on outdoor activities might be the reasons for vitamin D insufficiency.
In summary, our study showed the following results:
First, the levels of vitamin D in the serum of school-aged children in Korea could be determined. Although clinical symptoms such as rickets, bowing, growth retardation, and seizure were not found, vitamin D insufficiency was shown to be present in a high percentage of school-aged children in Korea. In addition, the greater the level of vitamin D insufficiency, the greater the BMI increased and the more advanced bone age for the chronological age was showed.
Second, after only mean 3.5 months of taking vitamin D, an increase in the level of 25-OH vitamin D3 in the serum of the children was seen, although the level was still less than 30 ng/mL. Therefore more research about vitamin D supplementation might be required.
Figures and Tables
Table 1
Characteristics of the Children according to the State of 25-OH Vitamin D3

Values are presented as number or mean±standard deviation.
25-OH vitamin D3: 25-hydroxy vitamin D, Weght (z): weight z-score, Height (z): height z-score, BMI: body mass index, BMI (z): BMI z-score, GV: growth velocity, RW: regular work (sun exposed time by regular work per week).
Statistic significance: p<0.05 by χ2 test.
References
2. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006; 81:353–373.

3. Kang B, Jung SY, Kim SK, Lee JE, Son BK, Kwon YS. Clinical features of seizures related to rickets in breastfed children. J Korean Child Neurol Soc. 2012; 20:179–187.
4. Cho HM, Choi CS, Sun GK, Kim EY, Kim KS, Kim YW. Two cases of rickets that developed as a result of by diet restriction due to atopic dermatitis. Korean J Pediatr Gastroenterol Nutr. 2006; 9:284–290.

5. Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008; 9:161–170.

6. Hatun S, Islam O, Cizmecioglu F, Kara B, Babaoglu K, Berk F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005; 135:218–222.

7. Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008; 162:513–519.

8. Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008; 18:145–150.

9. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84:18–28.

10. Yoon JH, Park CS, Seo JY, Choi YS, Ahn YM. Clinical characteristics and prevalence of vitamin D insufficiency in children less than two years of age. Korean J Pediatr. 2011; 54:298–303.

11. Kim MJ, Na B, No SJ, Han HS, Jeong EH, Lee W, et al. Nutritional status of vitamin D and the effect of vitamin D supplementation in Korean breast-fed infants. J Korean Med Sci. 2010; 25:83–89.

12. Yang HR, Seo JW, Kim YJ, Kim JY, Ryoo E, Sim JG, et al. Recent concepts on vitamin D in children and adolescents. Korean J Pediatr. 2009; 52:1082–1089.

13. Shin YH, Kim KE, Lee C, Shin HJ, Kang MS, Lee HR, et al. High prevalence of vitamin D insufficiency or deficiency in young adolescents in Korea. Eur J Pediatr. 2012; 171:1475–1480.

14. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004; 158:531–537.

15. Abrams SA. Dietary guidelines for calcium and vitamin D: a new era. Pediatrics. 2011; 127:566–568.

16. Neyestani TR, Hajifaraji M, Omidvar N, Eshraghian MR, Shariatzadeh N, Kalayi A, et al. High prevalence of vitamin D deficiency in school-age children in Tehran, 2008: a red alert. Public Health Nutr. 2012; 15:324–330.

17. Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007; 86:150–158.

18. Hickey L, Gordon CM. Vitamin D deficiency: new perspectives on an old disease. Curr Opin Endocrinol Diabetes. 2004; 11:18–25.

19. Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001; 86:1212–1221.

20. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011; 96:643–651.

21. Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr. 2011; 94:1020–1025.

22. Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009; 94:67–73.

23. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000; 72:690–693.

24. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008; 122:398–417.

25. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–1930.

26. Harkness LS, Cromer BA. Vitamin D deficiency in adolescent females. J Adolesc Health. 2005; 37:75.

27. Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr J. 2011; 10:51.

28. Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, et al. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res. 2006; 21:836–844.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download