Abstract
Corticosteroid (budesonide) nebulizer therapy is commonly performed. Its side effects have been considered as being safe or ignorable. The authors present a case of esophageal candidiasis in a healthy female adolescent who was treated with budesonide nebulizer therapy a few times for a cough during the previous winter season. This child presented with dysphagia and epigastric pain for 1 month. Esophageal endoscopy showed a whitish creamy pseudomembrane and erosions on the esophageal mucosa. Pathologic findings showed numerous candidal hyphae. She did not show any evidence of immunodeficiency, clinically and historically. The esophageal lesion did not resolve naturally. The esophageal lesion completely improved with the antifungal therapy for 2 weeks; the symptoms disappeared, and the patient returned to normal health. It is important that frequent esophageal exposure to topical corticosteroids application can cause unexpected side effects.
Recently, nebulizer therapy with short-acting corticosteroids such as budesonide or fluticasone is commonly being administered to or over-prescribed for pediatric patients presenting with a simple cough, even in private local pediatric clinics. Especially, in asthma patients, inhaled steroids are considered essential medication for their anti-inflammatory action [1]. Doctors think that locally-applied corticosteroid therapy is safe and causes less side effects compared with systemic corticosteroid therapy. But some adverse effects such as dysphagia, odynophagia, retrosternal pain, and hoarseness caused by local candidal infection in the esophagus of an adult patient were reported. The most common site of local candidal infections is the oro-pharynx, but a few cases of esophageal candidiasis have been reported [2,3]. The authors report a case of esophageal candidiasis in a female, previously healthy adolescent who had frequently received nebulizer therapy with budesonide a few times just for a cough.
A 13-year-old female adolescent visited our pediatric clinic presenting with nausea, dysphagia and abdominal pain. Her abdominal pain was localized to the epigastric area. The symptoms started 1 month ago, and became aggravated 2 weeks ago. The pain occurred intermittently, and became severe during the post-prandial period and at midnight, and was aggravated by a heavy meal. Her appetite was also decreased, but the body weight had not decreased. Her height was 152.3 cm (20th percentile), body weight was 41.6 kg (15th percentile), and body mass index was 17.9 (30th percentile). She had a history of frequent nebulizer therapy with budesonide for severe coughing at a private pediatric clinic although she had not been diagnosed with asthma or chronic bronchial disease. Every time she visited the pediatric clinic for the cough, she was prescribed nebulizer therapy with budesonide once a day for 2 or 3 consecutive days. This had occurred 3 times during the previous winter season. The dose of budesonide could not be confirmed. Her last budesonide nebulizer therapy was 2 months before she visited our clinic. Her history of use of antibiotics was unclear. The physical examination was nonspecific, except for mild epigastric tenderness. Blood studies were done, and they showed WBC 4,600/mm3 (polymorphonuclear neutrophils 54%, lymphocyte 36%, monocyte 7%), hemoglobin 12.9 g/dL, platelet 245,000/mm3, and C-reactive protein was negative. Electrolytes, total protein, albumin, calcium, phosphorus, cholesterol, liver enzymes, renal profiles, total bilirubin, direct bilirubin, CD3 and CD4 were within the normal range. In addition, serum Helicobacter pylori IgM and Ig G, and hepatitis B virus serologic tests were also negative, and human immunodeficiency virus was negative. Chest x-ray film showed no abnormality. Peptic ulcer disease was suspected clinically, so we planned an upper gastrointestinal endoscopic examination. On her first endoscopic examination, we found normal oral, gastric, and duodenal mucosa, but there were creamy white pseudo-membranes and multiple erosions on the whole of the esophageal mucosa (Fig. 1). Pathologic studies were performed on the esophageal biopsies of the lesion. KOH staining showed hyphae that suggested a fungal infection. Hematoxylin and eosin staining of the biopsy showed numerous hyphae and yeasts on the surface of the epithelia in the hyperkeratotic squamous epithelium of the esophagus (Fig. 2). Pathologically the lesion was consistent with candidal infection. So we were able to confirm candidiasis of esophagus. We thought that our patient was not in an immunocompromised state because she had no history of admission or operation, and was on no special medications except for budesonide. The patient's growth percentile was normal, and there was no immune-comprised clinical feature. So, we planned a wait-and-see approach without any antifungal treatment because the lesion could possibly heal naturally in an immunocompetent healthy host.
After 1 month, a follow-up esophagoscopic examination was performed. The previous whitish creamy pseudo-membranes and erosions were still noted on the whole esophagus (Fig. 3). We decided to prescribe an antifungal agent, oral fluconazole, that is usually well tolerated and effective for esophageal candidiasis, in the dose of 6 mg/kg per day for 2 weeks. As she took the medicine, her dysphagia, nausea, and abdominal pain improved gradually.
A follow-up esophagoscopic examination was performed 2 weeks after the cessation of the anti-fungal therapy. The whitish creamy pseudo-membranes and multiple erosions were no longer seen in the esophagus (Fig. 4). The esophageal mucosa was normal. Her symptoms also improved completely. She is now in excellent condition.
Infections of the esophagus are rare, and usually occur in immunocompromised patients. Known sources of esophageal infections are fungus, herpes simplex virus and cytomegalovirus [4]. Candida species are common cause of fungal esophagitis. Among these, the most common cause is Candida albicans, and others that have been found are Candida tropicalis, Candida krusei, Candida stellatoidea.
Fungal infections are controlled primarily by macrophages, lymphocytes and neutrophils. Immunosuppressive therapy, corticosteroids treatment (either systemic or local) targeting these cells, prolonged neutropenia, immunodeficiency diseases affecting neutrophil functions are important risk factors for fungal diseases. Prolonged treatment with broad-spectrum antibiotics is another risk factor. The natural bacterial flora is altered favoring increased fungal colonization. Other risk factors for esophageal candidiasis are endocrine diseases such as diabetes mellitus, hypothyroidism, hypoparathyroidism, malnutrition, chronic infectious disease, drug-induced suppression of gastric acid production, previous vagotomy, alcoholism, and advanced age [4,5].
This patient was previously healthy, received conventional medication for the coughing accompanied by nebulizer treatment during the short winter season, but she did not take long-term antibiotics. So, we concluded that frequent corticosteroid nebulizer treatment incurred the esophageal candidiasis.
Long-term use of inhaled corticosteroids may suppress the host's lymphocyte and granulocyte function, giving a chance for infective pathogens such as candida to grow. Candida has a higher chance of causing an infection than other pathogens because of its ubiquitous nature; it normally resides in the human's skin, oral cavity, gastrointestinal tract, and vagina [6].
The prevalence of esophageal candidiasis caused by the use of inhaled corticosteroids varies from 2.5% to 36.7% [1]. Kanda et al. [7] reported 5 cases of esophageal candidiasis in 18 diabetic patients who received inhaler steroid therapy. Shuto et al. [8] reported 7 cases of esophageal candidiasis in 20 asthma patients who were nebulized with fluticasone propionate dry powder. However, Mullaoglu et al. [1] reported that only a few cases developed esophageal and oropharyngeal candidiasis among the 40 asthma patients who had no risk factors and were treated with long-term inhaled corticosteroids, and similar cases of candidal colonization were noted in the control group as well, concluding that corticosteroid nebulizer therapy was safe. However, doctors need to be alert about this infectious side effect in the oro-esophageal mucosa after corticosteroid nebulizer therapy.
Because the group of patients with esophageal candidiasis induced by inhaled corticosteroids is small, there are no proven statistical reports about the significant relationship between the incidence of esophageal candidiasis and duration, type, dose and kind of inhaler device used. Furthermore, all these cases were reported in adult patients, and not in children.
There were two cases of esophageal candidiasis developing in immunocompetent childen [9,10]. One case was an 11-year-girl with chronic epigastric pain, vomiting and headache for 4 months. She had no specific past history and there was no abnormality in the immunologic examinations. She had no history of corticosteroid inhalation therapy. No other risk factor was found in this case [9]. The other case was an 18-month-old girl, who presented with effortless vomiting associated with coffee-ground emesis and melena for 1 day. She was prematurely born, had grown well, and had one history of respiratory infection with respiratory syncytial virus at 10 months of age when she was diagnosed with asthma, and she was treated with a fluticasone inhaler. Her immune function was normal, and she had no risk factors for esophageal candidiasis [10].
The first step for treatment of esophageal candidiasis is to minimize all the possible risk factors, such as corticosteroids, chemotherapeutic agents, and antimicrobials. Antifungal therapy for esophageal candidiasis include topical antifungal agents such as nystatin, clotrimazole, and miconazole, oral or parenteral fluconazole, flucytosine, itraconazole, and amphotericin B [11]. Topical antifungal agents such as nystatin are known to be ineffective in the treatment of esophageal candidiasis. Oral fluconazole for 14 days has been the treatment of choice for esophageal candidiasis because it has safety compared with ketoconazole, excellent gastric absorption, and can be used intravenously. Moreover, fluconazole has the characteristics of rapid onset of action and producing fast resolution of symptoms. Itraconazole has also been known to be effective in the treatment of esophageal candidiasis [3]. There seems to be no case report of natural healing of esophageal candidiasis without any treatment in a healthy host.
There are no special methods of prevention of esophageal candidiasis for the inhaled corticosteroid user, but we think that general hygienic techniques like the use of disposable kits and a wash-out method will help to avoid esophageal candidiasis.
It would have been ideal to have attained further results in the form of serum immunoglobulins, T-cell subset, serologic cytomegalovirus and herpes simplex virus infection, and lymphokines to check for immunodeficiency and to differentiate from esophageal viral infections. But at that time, these tests could not be performed due to the financial limitations of the family.
In conclusion, we described an adolescent with normal immunity who was diagnosed with esophageal candidiasis caused by frequent use of corticosteroid nebulizer therapy. Therefore, most pediatricians who perform nebulizer therapy for many children and believe there would be no specific side effects induced by this therapy need to be aware that inhaled corticosteroids can incur unexpected side effects such as esophageal candidiasis even in an immunocompetent host.
Figures and Tables
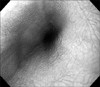
Fig. 1
Esophageal endoscopic finding shows creamy whitish pseudo-membranes and erosions on the whole esophageal mucosa.

Fig. 2
The endoscopic biopsy revealed many fungal organisms (arrows) with hyphae morphology in the hyperkeratotic squamous epithelium of the esophagus. Morphologically, this was consistent with Candida species (hematoxylin and eosin stain, ×400).

References
1. Mullaoglu S, Turktas H, Kokturk N, Tuncer C, Kalkanci A, Kustimur S. Esophageal candidiasis and Candida colonization in asthma patients on inhaled steroids. Allergy Asthma Proc. 2007; 28:544–549.

2. Aun MV, Ribeiro MR, Costa Garcia CL, Agondi RC, Kalil J, Giavina-Bianchi P. Esophageal candidiasis--an adverse effect of inhaled corticosteroids therapy. J Asthma. 2009; 46:399–401.

3. Vazquez JA. Optimal management of oropharyngeal and esophageal candidiasis in patients living with HIV infection. HIV AIDS (Auckl). 2010; 2:89–101.

4. Misra S, Ament ME. Esophagitis. In : Feigin RD, Cherry JD, editors. Textbook of pediatric infectious diseases. 4th ed. Philadelphia: Saunders;1997. p. 562–566.
5. Kliemann DA, Pasqualotto AC, Falavigna M, Giaretta T, Severo LC. Candida esophagitis: species distribution and risk factors for infection. Rev Inst Med Trop Sao Paulo. 2008; 50:261–263.

7. Kanda N, Yasuba H, Takahashi T, Mizuhara Y, Yamazaki S, Imada Y, et al. Prevalence of esophageal candidiasis among patients treated with inhaled fluticasone propionate. Am J Gastroenterol. 2003; 98:2146–2148.

8. Shuto H, Nagata M, Terashi Y, Yamaguchi M, Takizawa T, Shuto C, et al. Esophageal candidiasis as complication of inhaled steroid therapy. Arerugi. 2003; 52:1053–1064.
9. Kim YJ, Jang SJ. Esophagus, stomach & intestine; a case of esophageal candidiasis presenting recurrent abdominal pain in an immunocompetent child. Korean J Gastrointest Endosc. 1997; 17:55–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download