INTRODUCTION
Therapeutic strategies aiming for disease control of CD are evolving, owing not only to updated algorithms aiming for clinical remission, but also to new medications that are building a solid armamentarium to treat IBD.
1 The natural history of CD often leads to bowel damage (stenosis and/or perforation) if inflammation is not controlled.
2 Despite the development, approval, and increase in the use of biological therapies in the management of CD and evolution to the loss of response to medical therapy, stenosis and fistulas can still occur; further, major intestinal resections can still often be necessary in a significant proportion of patients.
3
Data from population-based studies showed a tendency of a reduction in surgical rates in CD, which was remarkably noticed after the biological era. In a study from Whales (Cardiff area), the 5-year probability of surgery decreased over the period of diagnosis of CD, ranging from 59% to 25% in the most recent years of the study.
4 In a similar way, data from Denmark demonstrated a reduction in surgical rates over different study periods. The 10-year cumulative proportion of surgery in patients with CD diagnosed from 1979 to 1986 was 50.3%, which dropped to 23.3% in the patients diagnosed from 2003 to 2011.
5 As the use of biological agents represented the most significant change in IBD practice over the last 2 decades, they are probably related to this significant decrease observed in several studies.
6
This could also be observed in prospective studies on biological agents. The REACT-1 trial demonstrated that major adverse outcomes (need for surgery included) decreased in patients treated with early combined immunosuppression as compared with those in patients treated with conventional management.
1 Data from the CHARM trial with adalimumab (ADA) also demonstrated a significant reduction in the need for surgery in a 1-year period in CD (0.6% for ADA as compared with 3.8% for placebo,
P<0.001).
7 A recent meta-analysis demonstrated that the OR for the reduction of surgery using biologics in prospective trials was 0.23 (95% CI, 0.13−0.42), showing a protective effect of these agents with regards to the need for major surgical resections.
8
Despite the evidence that biologics reduce the surgical rates over time, this still needs to be reflected in clinical practice. Whether the use of anti-tumor necrosis factor (anti-TNF) agents reduces or delays the indication for surgery in some patients remains a matter of debate. The duration of the disease from diagnosis to surgery can also be affected by medical therapy. Some additional data from different parts of the world also demonstrated that patients receiving biologics have longer intervals until the need for a surgical resection.
910 Indeed, early anti-TNF initiation also increased the time to intestinal resection as compared with late initiation in other retrospective studies.
1112 Other factors, such as smoking, can also influence the timing of the need for a surgical resection.
13
The primary aim of this study was to determine the influence of biological therapy on the time from diagnosis to the need for an elective surgical resection in a cohort of Brazilian patients with CD. The secondary aim was to outline the impact of the specific type of anti-TNF agent (infliximab [IFX] or ADA) and preoperative use of steroids and immunomodulators (azathioprine [AZA] or 6-mercaptopurine [6-MP]), as well as smoking and perianal CD on the interval of the time to surgery.
METHODS
1. Study Design
This was a retrospective observational cohort study that included patients with CD submitted to intestinal resections due to complications (stenosis or penetrating disease) or medical therapy failure from 2 different IBD centers in Brazil in a 7-year period (January 2007−July 2014).
2. Inclusion and Exclusion Criteria
Patients with an established diagnosis of CD according to symptoms, imaging, endoscopic, and histological criteria, aging over 18 years old at the time of surgery, and submitted to any elective abdominal surgical procedure with intestinal resection were included in the study. We excluded patients with undetermined colitis, those submitted to strictureplasties or abdominal procedures without intestinal resection (diverting stomas or non-intestinal abdominal surgeries), and those operated in the emergency setting.
3. Variables of Interest
The following variables were analyzed: age at diagnosis, age at surgery, gender, time from diagnosis to surgical procedure, and smoking status. The patients' phenotype was described in accordance with the Montreal classification. Perianal disease and preoperative medication use (steroids, immunomodulators, and/or biological agents) were also checked.
Regarding preoperative exposure to medications, the patients with previous anti-TNF agent (IFX or ADA) use of up to 8 weeks from the surgical procedure were considered exposed. Previous steroid and thiopurine use was also checked. The specific protocols with the variables previously described were retrospectively completed after electronic chart review.
4. Definition of the Study Groups
After patient identification and selection, they were allocated into 2 groups according to their previous exposure to anti-TNF agents (ADA or IFX) in the preoperative period. The patients with conventional therapy included those who received AZA 2.0 to 2.5 mg/kg/day or 6-MP 1.0 to 1.5 mg/kg/day. The patients in the anti-TNF group had regular induction and maintenance dosages (IFX 5 mg/kg at weeks 0, 2, and 6 and every 8 weeks; ADA 160/80 mg at weeks 0 and 2 and 40 mg every 2 weeks). The indications for anti-TNF therapy in our country are mostly based on medical therapy failure defined as the persistence of symptomatic active disease after 6 months of use of immunomodulators (AZA or 6-MP), according to the local reimbursement rules. Dose optimization of anti-TNF agents could be used according to physicians' perception empirically, as therapeutic drug monitoring is not currently performed in our country. The patients with preoperative biologics were then compared with those treated with conventional therapy, regarding the time from diagnosis to surgery and other possible factors that could influence such. The same analysis regarding the previous use of AZA/6-MP and steroids as well as the smoking status and presence of perianal CD was performed in the patients.
5. Statistical Analysis
The results were expressed as medians and standard deviations. For the analysis of group homogeneity, in relation to the quantitative variables, the Student t-test (normal distribution) or Mann-Whitney U-test (non-parametric distribution) was used. For the qualitative variables, the Fisher exact test and the chi-square method were selected. Kaplan-Meier survival analyses were conducted for the event of surgery during the disease course between the groups. A Cox regression analysis was used to identify the predictors of the variables. P-values of <0.05 were considered significant. The analysis was performed using the SPSS version 14.0 software (SPSS Inc., Chicago, IL, USA).
6. Ethical Aspects
The study was approved by the ethical boards of the Catholic University of Parana and Campinas State University, according to protocol 63324/2012 and CAAE 03687612.8.1001.5404, from the Ministry of Health website “plataforma Brasil.” This study was waived for informed consent.
RESULTS
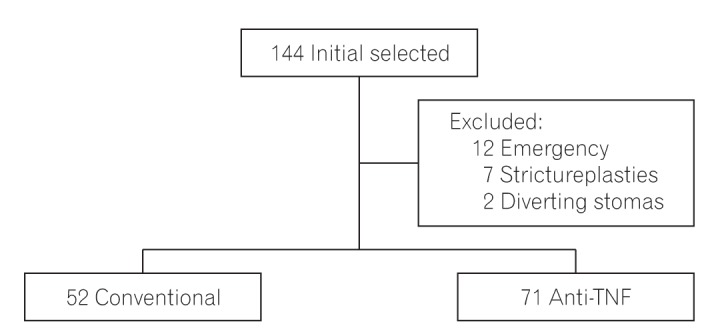
Initially, 144 patients were identified from the surgical databases of both units, and 21 were excluded owing to different reasons. From the 12 patients excluded owing to emergency surgeries, 9 were on conventional therapy and probably had a delay in accessing biological therapy as a proper treatment, thereby consequently developing urgent complications; they were excluded to reduce possible bias. Therefore, 123 patients comprised the final sample of our study (
Fig. 1). From this sample, 52 were preoperatively treated with conventional therapy, while 71 received anti-TNF agents (39 on IFX and 32 on ADA). From the 32 patients under ADA therapy, 13 had previously used IFX.
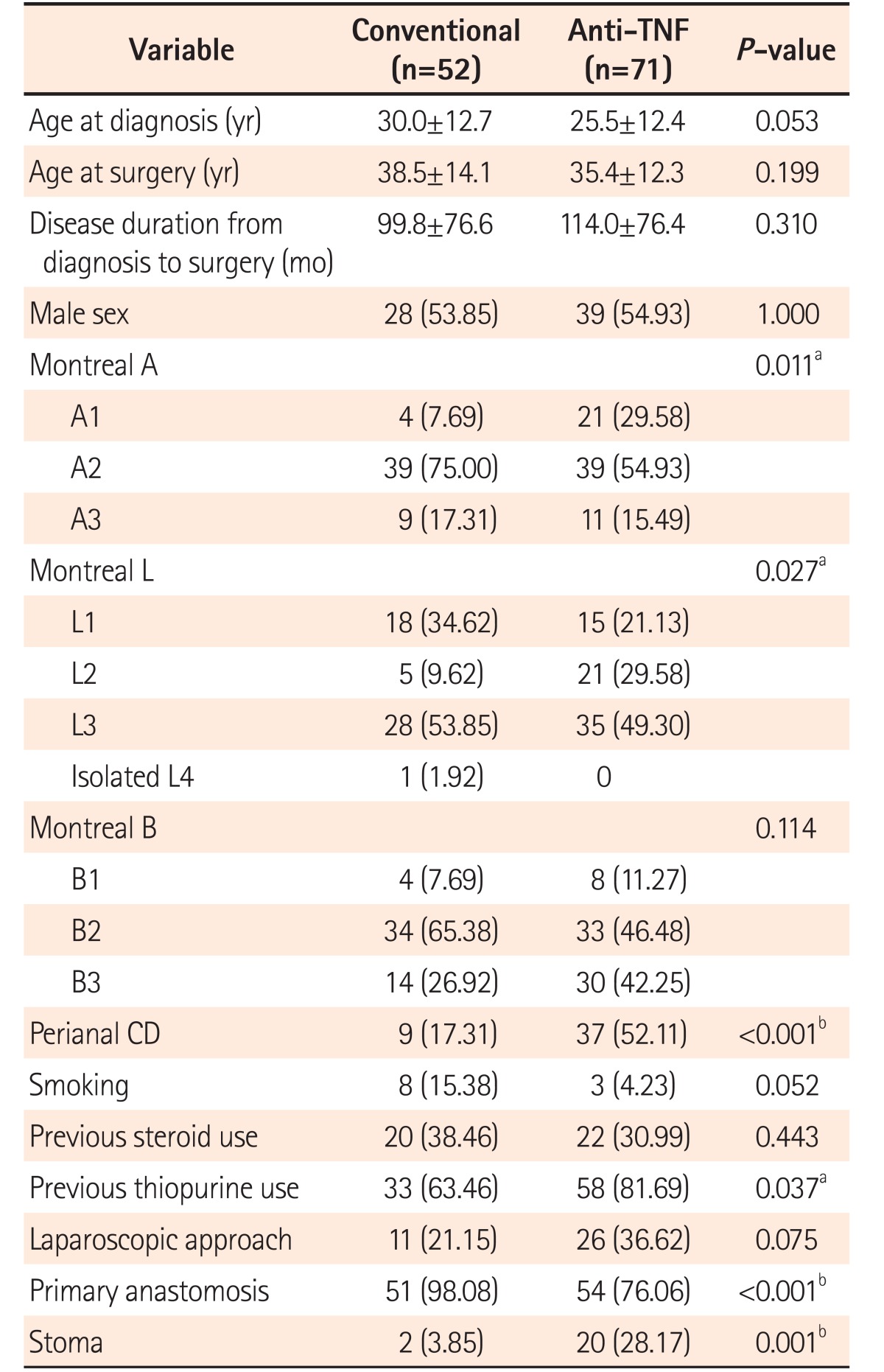
The baseline characteristics of the patients, according to the study groups, are described in detail in
Table 1. There was no significant difference between the groups in terms of gender, age at diagnosis, age at surgery, time from diagnosis to procedure, smoking, hypoalbuminemia, and previous steroid use. However, the groups were not fully homogeneous. As observed, the patients with previous anti-TNF use were predominantly younger (Montreal A1) and had colonic location (Montreal L2) with perianal disease and more exposure to previous thiopurines than those under conventional treatment. Moreover, in terms of surgical characteristics, the patients in the anti-TNF group also had more stomas created and less primary anastomoses than the patients in the control group.
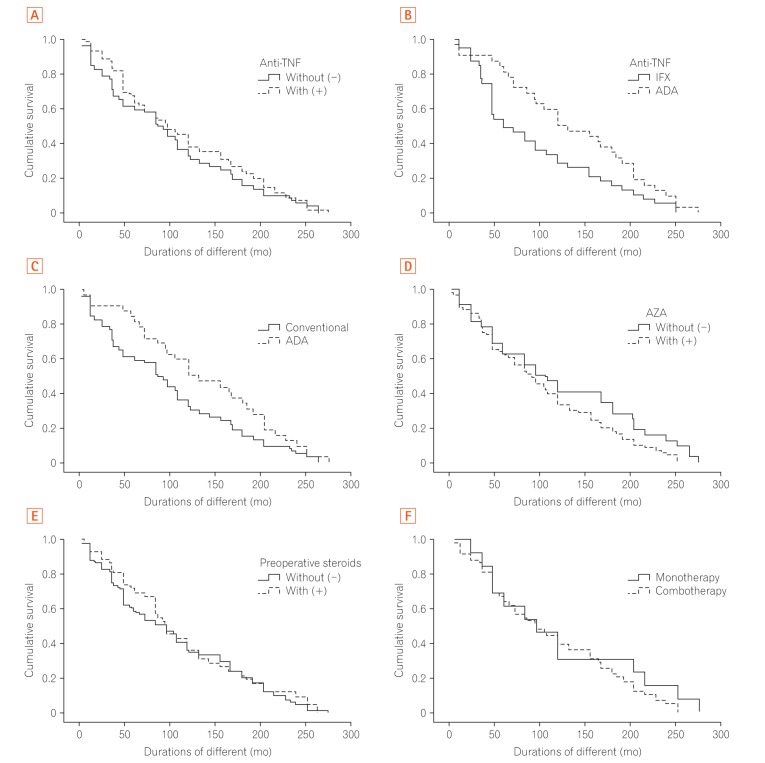
Regarding the primary outcome of our study, there was no difference in the interval from diagnosis to surgery in the patients who used anti-TNFs as compared with those under conventional therapy (99.78±10.62 months in the patients without and 114.01±9.07 months in the patients with previous anti-TNF use). The absolute duration was longer in the biologics group; however, it was not statistically significant (log-rank
P=0.35). The time to surgery between the 2 groups is illustrated in
Fig. 2A. Aiming to evaluate if age at surgery affected the time from diagnosis to surgery, the Cox regression model demonstrated no difference between the groups regarding exposure to anti-TNF agents (β=1.008,
P=0.28).
Regarding the specific type of anti-TNF agent used, the patients under IFX therapy had a shorter interval from diagnosis to surgery (94.76±11.41 months) than those under ADA therapy (137.46±13.61 months), with a statistically significant difference (log-rank
P=0.034) as illustrated in
Fig. 2B. There was no significant difference in the comparison between the patients who used ADA (137.46±13.61 months) and conventional therapy (102.74±7.56 months) (log-rank
P=0.69) as illustrated in
Fig. 2C.
In terms of conventional therapy, no significant difference was found in the time to surgery between the patients with and without AZA use (102.74±7.56 months vs. 122.93±15.41 months, respectively, log-rank
P=0.073) as illustrated in
Fig. 2D. Additionally, the patients who used steroids (
Fig. 2E) before surgery also did not have a different interval until surgery compared with those who did not (113.14±11.56 months vs. 105.33±8.60 months, respectively, log-rank
P=0.58). No difference was also found between the patients on monotherapy (n=13, 39.53 months) and combotherapy (n=58, 34.53 months) (log-rank
P=0.35) (
Fig. 2F).
Regarding the smoking status, no significant difference was found in the time to surgery. The smokers and non-smokers had an interval from diagnosis to surgery of 106.00±29.98 and 108.19±7.03 months, respectively (log-rank P=0.63). The presence of perianal CD also did not influence the time from diagnosis to surgery. The patients with and without perianal CD had an interval from diagnosis to surgery of 118.60±10.89 and 101.66±8.90 months, respectively (log-rank P=0.49).
DISCUSSION
Surgery is still an important tool in IBD management. The rates of surgical treatment are even higher in CD than in UC, possibly owing to the transmural course of the disease, often leading to fibrotic stenosis and internal or external fistulas, with clear surgical indications. Several population-based studies
456 and sub-analysis of prospective trials
7 have demonstrated that the surgical rates are decreasing in the biological era, with the widespread use of anti-TNF agents. Anti-TNF therapy, if properly used, can alter the natural course of the disease, aiming for mucosal healing and disease control and avoiding the evolution to bowel damage that often leads to surgery. Moreover, some studies have demonstrated that patients with anti-TNF therapy have longer intervals from diagnosis to the first surgery, showing that in those cases they cannot avoid, they often delay a surgical procedure in determined patients.
910
The results of the survival analysis in our study demonstrated an absolute difference of 15 months in the time from diagnosis to surgery, regarding the status of the previous use of anti-TNF therapy in the cohort of patients. The patients who used IFX or ADA had a median interval from diagnosis to surgery of 114.01 months, whereas those with conventional therapy had an interval of 99.78 months. Despite the absolute difference in the numbers, no statistical significance in that analysis was found (log-rank
P=0.35), which was probably because of the small sample size of the patients in the 2 groups. This absence of difference can also be explained by the observational design of our study and the heterogeneity of the patients. In a recent systematic review, Olivera et al.
6 demonstrated the trend of reduced surgical rates in several population-based studies and few randomized controlled trials. The authors stated that the reduction in surgeries was already observed before the biological era, and the use of anti-TNF agents between 2000 and 2010 had probably contributed to this trend.
In our analysis, the ADA patients had surgery later than the IFX patients (time from diagnosis to surgery of 137.46 months vs. 94.76 months, respectively). We did not perform a multivariate analysis to determine whether ADA could independently influence the time to surgery. This difference may be clearly guided by the selection bias; as in the clinical practice, patients with more severe conditions tend to receive IFX as their initial therapy and undergo surgery earlier. Moreover, 40.6% of the ADA patients previously used IFX, which could also have influenced these results. There are data from clinical trials demonstrating that both agents can reduce surgical rates.
714 However, no studies have demonstrated head-to-head comparisons between ADA and IFX in terms of time from diagnosis to surgery; thus, this needs further prospective investigations. Moreover, no data from other biological agents, such as vedolizumab or ustekinumab, and their relation to a reduction or a delay in surgery rates were published to date.
Conventional therapy, mainly including steroids and AZA/6-MP, did not significantly influence the interval from diagnosis to surgery in our cohort of patients. Regarding steroid use, it is clear that steroids usually tend to improve symptoms, but are not disease-modifying agents. Therefore, aiming for a better symptom control in patients from long waiting lists for surgery may justify the use of steroids in clinical practice, even when surgery has been properly indicated.
The role of thiopurines in preventing or delaying surgery is controversial in the international literature. In a classic study in 2005 in France, Cosnes et al.
15 demonstrated that despite the increase in the use of immunomodulators, such as AZA and 6-MP, over the years, there was no change in the rates of abdominal surgeries in patients with CD. This was based on the findings of our study, where AZA/6-MP did not significantly alter the survival curves until surgery in comparison with those of the patients who did not use this medication. Other studies reported conflicting results, stating that there can be a significant reduction in surgical rates or delay in surgery indication in patients receiving thiopurines.
16 However, the power of disease modification of thiopurines and the impact they cause on surgical rates are considered to be currently under control, according to 2 negative prospective studies on the efficacy of such in patients with CD.
1718
In our observational cohort of patients, perianal CD and smoking also did not influence the time to surgical resection. Perianal CD is usually a marker of more severe disease, and as a coincidental factor, it could be associated with patients who need surgery. This may be observed in a larger study population, as our cohort only analyzed 123 surgical patients. A recent systematic review and meta-analysis conducted in Canada demonstrated that the time from diagnosis to surgery was shorter in smokers until a primary intestinal resection due to CD.
13 Our study did not observe such a link, probably owing to the reduced number of smokers in our sample (only 11 from the 123 patients analyzed), cultural trend in our country, and intense campaigns and public health efforts against smoking.
Our study has some limitations that must be taken into account when analyzing our results. First, all biases regarding the retrospective observational design, with data collection impairments, should be considered. Moreover, the survival analysis could be more accurate if analyzed prospectively. The groups were not fully homogeneous as what could be expected in an observational study when reflecting actual practice; patients under biological therapy tend to have some characteristics that reflect clear signs of more severe disease (e.g., early age at diagnosis). Moreover, we did not perform sample size calculation, as all patients were initially checked (convenience sample) during the study period, and the study could be underpowered to detect a difference between the groups. Another important limitation was that the initial disease activity could not be evaluated to determine whether the patients under biologics had more severe disease than those under conventional therapy. Although Brazilian physicians usually follow the European Crohn's and Colitis Organisation guidelines,
19 no strict standardized strategy was used for all patients. Nevertheless, the strength of our study was that we reported the first data regarding the time from diagnosis to surgery in Latin American patients and its relation to medication use in general.
In summary, the time from diagnosis to surgery was not influenced by the preoperative use of anti-TNF therapy in this cohort of patients. The patients who used ADA tended to have a longer time from diagnosis to surgery than those who used IFX. There was no impact of previous steroid and thiopurine use, perianal CD, and smoking on the time from diagnosis to intestinal resection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download