1. Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013; 48:287–302. PMID:
23208018.
2. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012; 107:1315–1329. PMID:
22710576.

3. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011; 42:1–10. PMID:
20869746.
4. Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009; 4:343–364. PMID:
19400693.

5. Bauer VP, Papaconstantinou HT. Management of serrated adenomas and hyperplastic polyps. Clin Colon Rectal Surg. 2008; 21:273–279. PMID:
20011438.
6. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010; 138:2088–2100. PMID:
20420948.
7. Huang CS, Farraye FA, Yang S, O'Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2011; 106:229–240. PMID:
21045813.
8. Aust DE, Baretton GB. Members of the Working Group GI-Pathology of the German Society of Pathology. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010; 457:291–297. PMID:
20617338.
9. Sweetser S, Smyrk TC, Sugumar A. Serrated polyps: critical precursors to colorectal cancer. Expert Rev Gastroenterol Hepatol. 2011; 5:627–635. PMID:
21910580.
10. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013; 369:1106–1114. PMID:
24047060.
11. le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014; 63:957–963. PMID:
23744612.

12. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993; 329:1977–1981. PMID:
8247072.

13. Lieberman DA. American Gastroenterological Association. Colon polyp surveillance: clinical decision tool. Gastroenterology. 2014; 146:305–306. PMID:
24269291.

14. Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013; 45:842–851. PMID:
24030244.

15. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143:844–857. PMID:
22763141.

16. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorec-tal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010; 59:666–689. PMID:
20427401.

17. British Columbia Medical Association. BCGuidelines.ca: follow-up of colorectal polyps or cancer. Victoria: British Columbia Ministry of Health;2013.
19. Baron TH, Smyrk TC, Rex DK. Recommended intervals between screening and surveillance colonoscopies. Mayo Clin Proc. 2013; 88:854–858. PMID:
23910411.

20. Buda A, De Bona M, Dotti I, et al. Prevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopy. Clin Transl Gastroenterol. 2012; 3:e6. DOI:
10.1038/ctg.2011.5. PMID:
23238028.

21. IJspeert JE, de Wit K, van der Vlugt M, Bastiaansen BA, Fockens P, Dekker E. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy. 2016; 48:740–746. PMID:
27110696.

22. Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009; 104:695–702. PMID:
19223889.

23. Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010; 139:1497–1502. PMID:
20633561.

24. Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010; 139:1503–1510. PMID:
20643134.

25. Lu FI, van Niekerk de W, Owen D, Tha SP, Turbin DA, Webber DL. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010; 34:927–934. PMID:
20551824.

26. Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011; 54:1216–1223. PMID:
21904135.

27. Glazer E, Golla V, Forman R, Zhu H, Levi G, Bodenheimer HC Jr. Serrated adenoma is a risk factor for subsequent adenomatous polyps. Dig Dis Sci. 2008; 53:2204–2207. PMID:
18320324.

28. Álvarez C, Andreu M, Castells A, et al. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013; 78:333–341.e1. PMID:
23623039.

29. Zhu H, Zhang G, Yi X, et al. Histology subtypes and polyp size are associated with synchronous colorectal carcinoma of colorectal serrated polyps: a study of 499 serrated polyps. Am J Cancer Res. 2014; 5:363–374. PMID:
25628945.
30. Leung WK, Tang V, Lui PC. Detection rates of proximal or large serrated polyps in Chinese patients undergoing screening colonoscopy. J Dig Dis. 2012; 13:466–471. PMID:
22908972.

31. Lazarus R, Junttila OE, Karttunen TJ, Mäkinen MJ. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005; 123:349–359. PMID:
15716230.

32. Seo JY, Choi SH, Chun J, et al. Characteristics and outcomes of endoscopically resected colorectal cancers that arose from sessile serrated adenomas and traditional serrated adenomas. Intest Res. 2016; 14:270–279. PMID:
27433150.

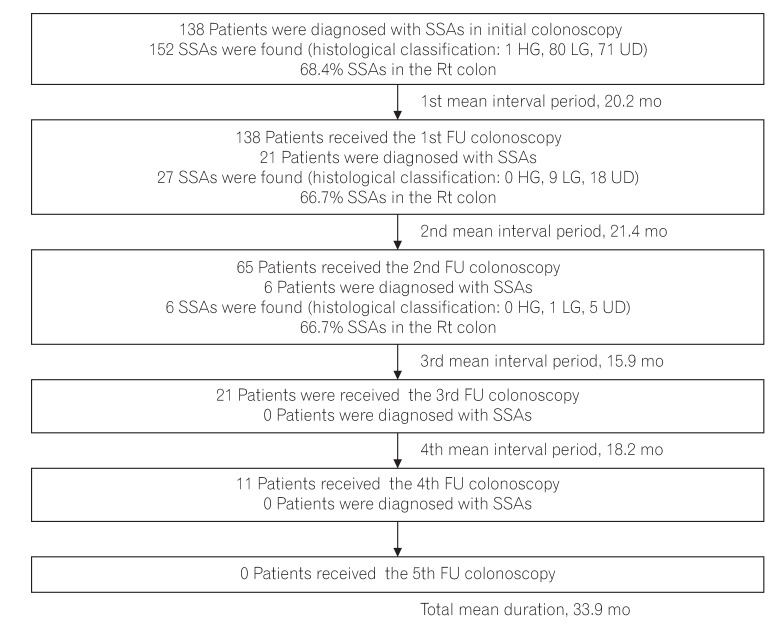
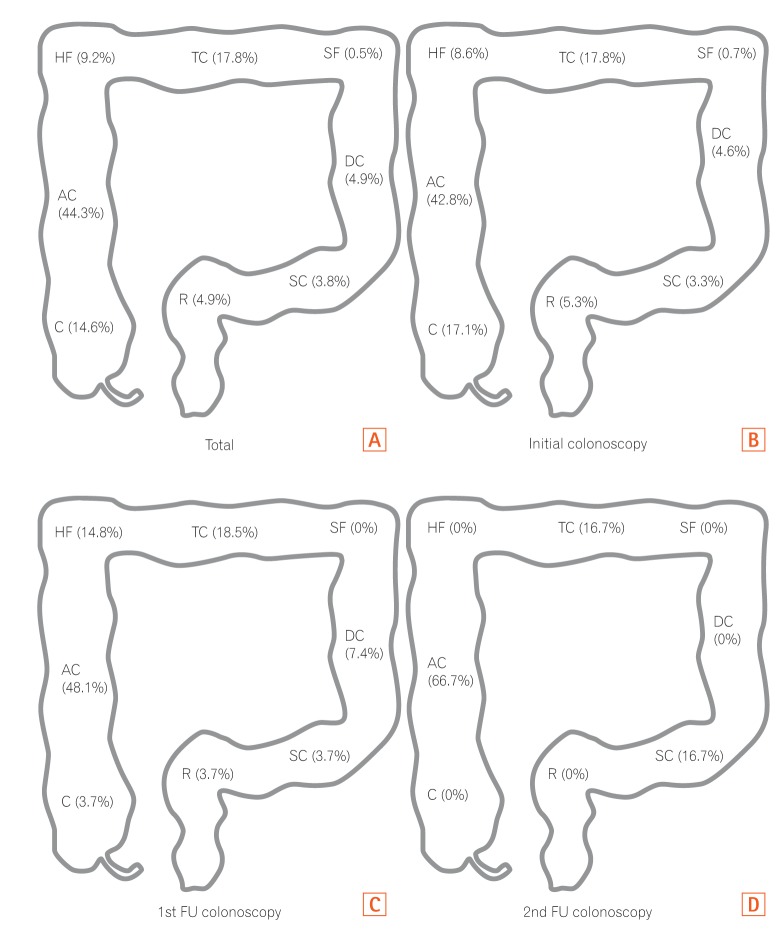
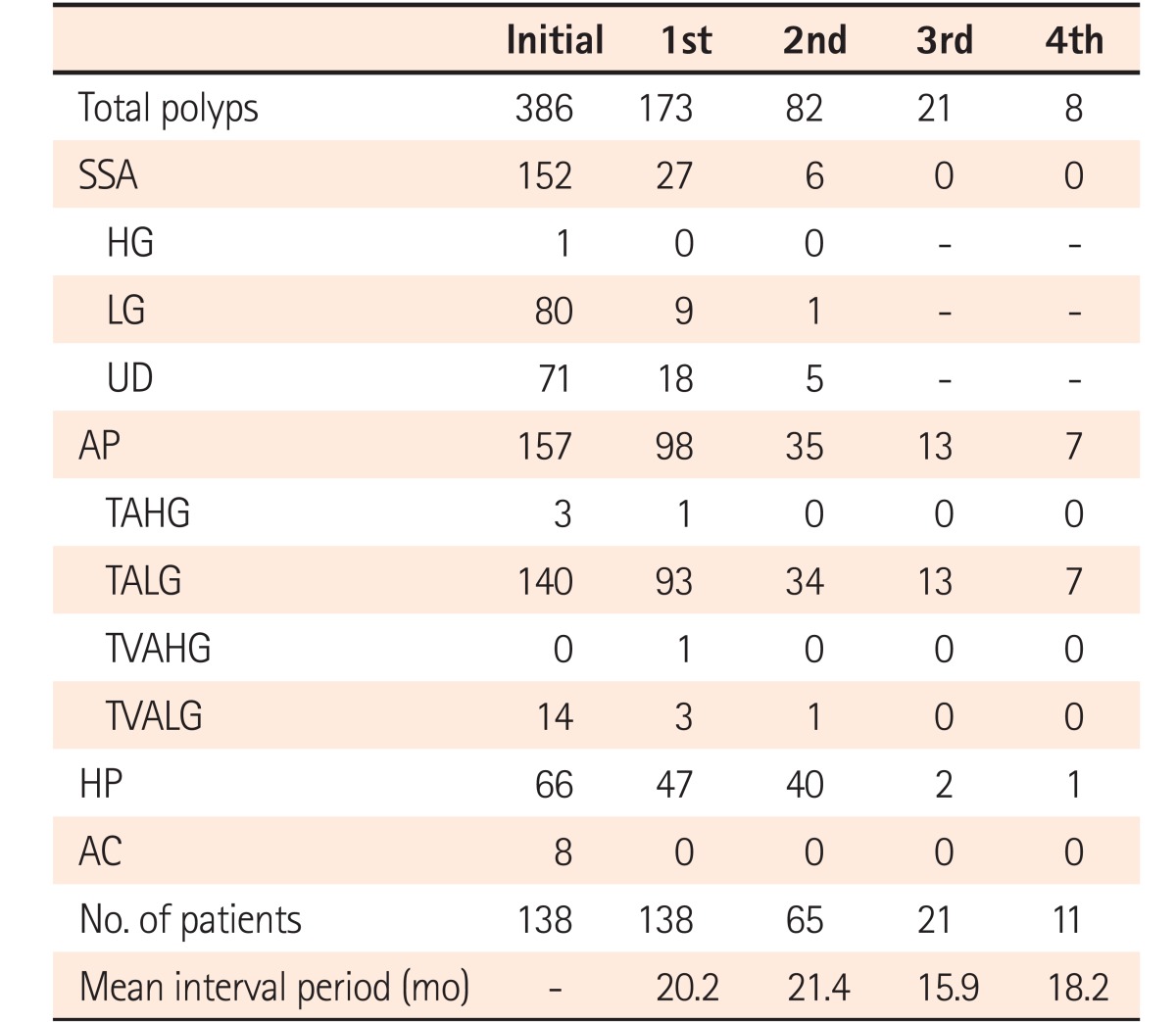
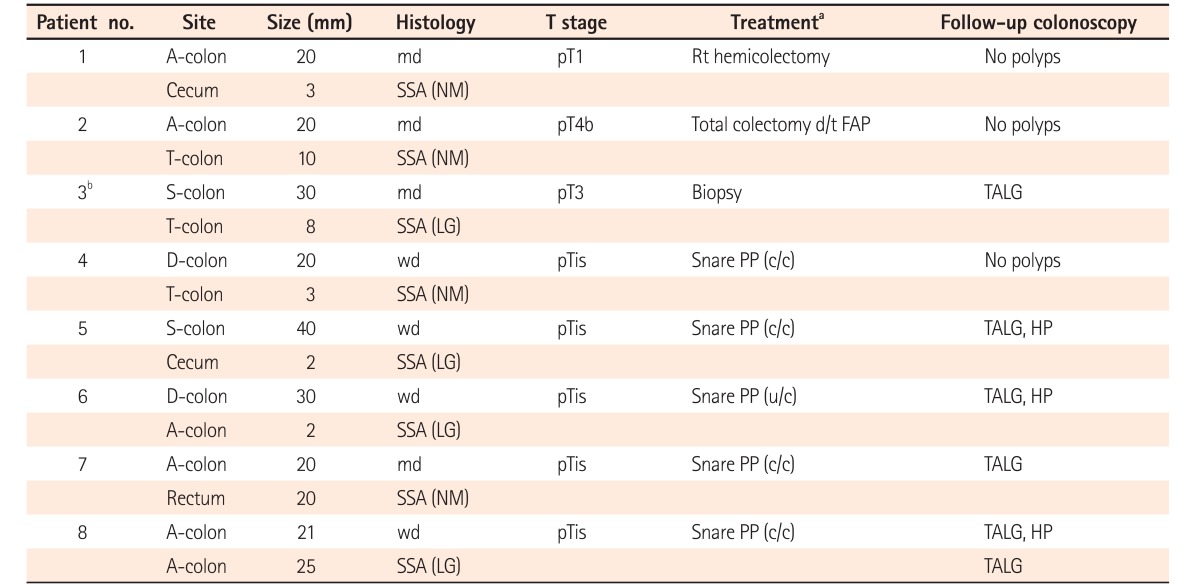




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download