1. Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015; 64:57–65. PMID:
24986245.

2. Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009; 70:344–349. PMID:
19249767.

3. Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015; 110:697–707. PMID:
25848926.

4. Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001; 54:62–66. PMID:
11427843.

5. Yang DH, Jeong GH, Song Y, et al. The feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissection. Dig Dis Sci. 2015; 60:3431–3441. PMID:
26088371.

6. Woodward TA, Heckman MG, Cleveland P, De Melo S, Raimondo M, Wallace M. Predictors of complete endoscopic mucosal resection of flat and depressed gastrointestinal neoplasia of the colon. Am J Gastroenterol. 2012; 107:650–654. PMID:
22552236.

7. Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010; 24:343–352. PMID:
19517168.

8. Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012; 26:2220–2230. PMID:
22278105.

9. Tajika M, Niwa Y, Bhatia V, et al. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011; 23:1042–1049. PMID:
21869682.

10. Byeon JS, Yang DH, Kim KJ, et al. Endoscopic submucosal dissection with or without snaring for colorectal neoplasms. Gastrointest Endosc. 2011; 74:1075–1083. PMID:
21663905.
11. Hong YM, Kim HW, Park SB, Choi CW, Kang DH. Endoscopic mucosal resection with circumferential incision for the treatment of large sessile polyps and laterally spreading tumors of the colorectum. Clin Endosc. 2015; 48:52–58. PMID:
25674527.
12. Sakamoto T, Matsuda T, Nakajima T, Saito Y. Efficacy of endoscopic mucosal resection with circumferential incision for patients with large colorectal tumors. Clin Gastroenterol Hepatol. 2012; 10:22–26. PMID:
22016034.
13. Kim HG, Sethi S, Banerjee S, Friedland S. Outcomes of endoscopic treatment of second recurrences of large nonpedunculated colorectal adenomas. Surg Endosc. 2016; 30:2457–2464. PMID:
26423413.
14. Sakamoto T, Saito Y, Matsuda T, Fukunaga S, Nakajima T, Fujii T. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011; 25:255–260. PMID:
20559661.
15. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255. PMID:
10896917.

16. Seo GJ, Sohn DK, Han KS, et al. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol. 2010; 16:2806–2811. PMID:
20533602.

17. Hotta K, Fujii T, Saito Y, Matsuda T. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis. 2009; 24:225–230. PMID:
18972121.

18. Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014; 46:388–402. PMID:
24671869.

19. Sakamoto T, Matsuda T, Otake Y, Nakajima T, Saito Y. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol. 2012; 47:635–640. PMID:
22223177.

20. Regula J, Wronska E, Polkowski M, et al. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: long-term follow-up study. Endoscopy. 2003; 35:212–218. PMID:
12584639.

21. Tsiamoulos ZP, Bourikas LA, Saunders BP. Endoscopic mucosal ablation: a new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc. 2012; 75:400–404. PMID:
22154411.

22. Holmes I, Kim HG, Yang DH, Friedland S. Avulsion is superior to argon plasma coagulation for treatment of visible residual neoplasia during EMR of colorectal polyps (with videos). Gastrointest Endosc. 2016; 84:822–829. PMID:
27080417.

23. Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014; 80:1094–1102. PMID:
25012560.

24. Kim EK, Han DS, Ro Y, Eun CS, Yoo KS, Oh YH. The submucosal fibrosis: what does it mean for colorectal endoscopic submucosal dissection? Intest Res. 2016; 14:358–364. PMID:
27799887.

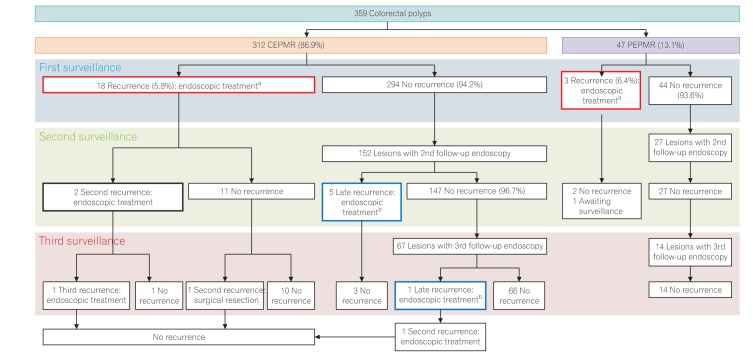
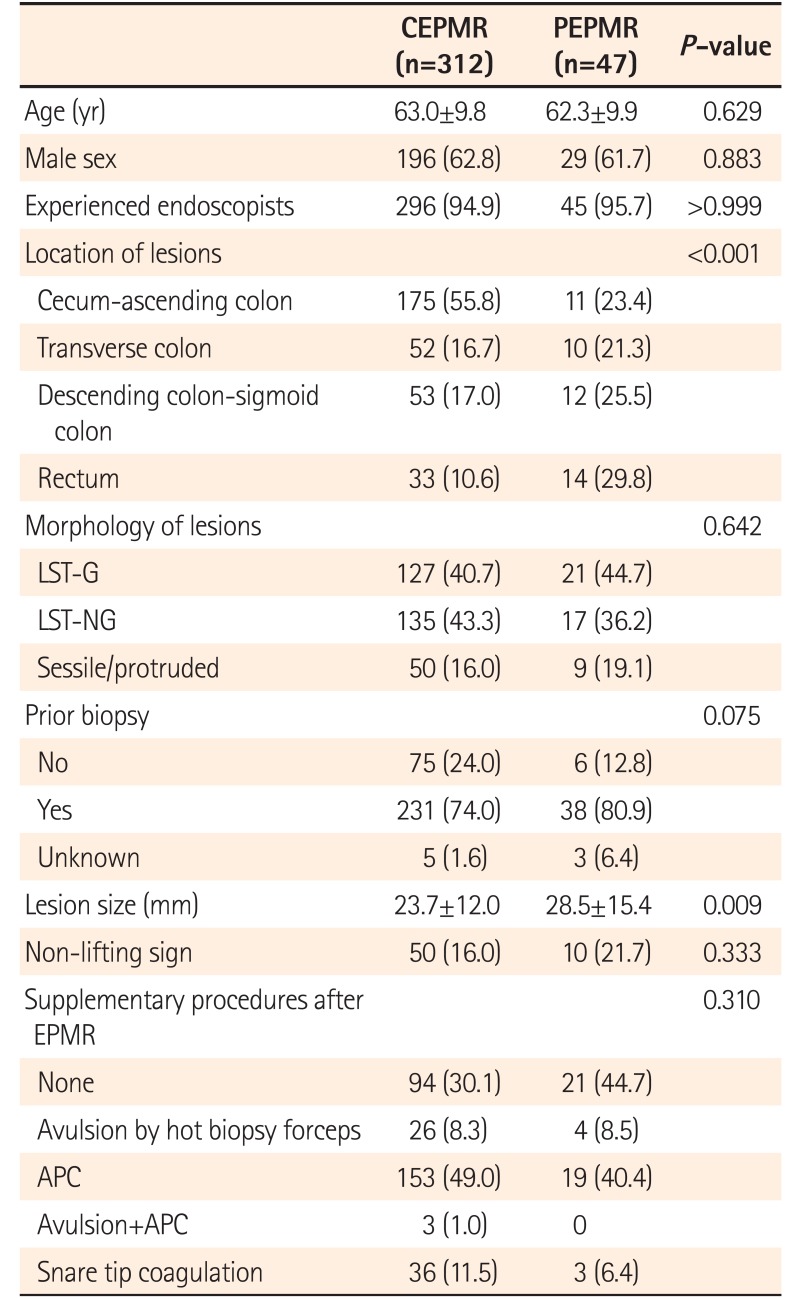
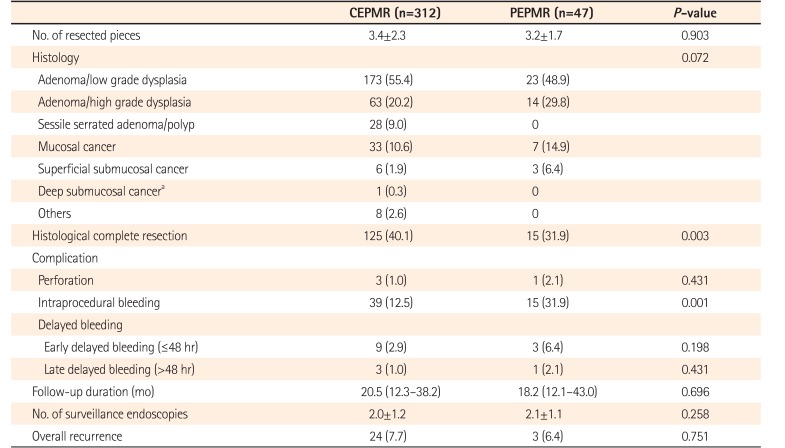
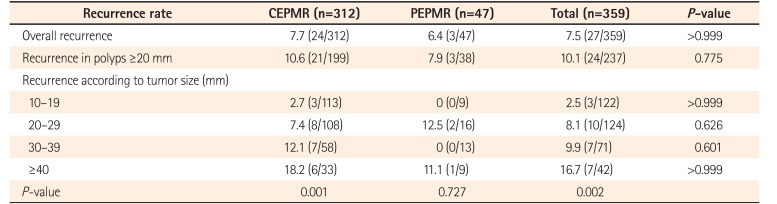




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download