Abstract
Background/Aims
Recent developments in analytical techniques including next-generation sequencing have clarified the correlation between intestinal microbiota and inflammatory bowel disease. Fecal microbiota transplantation (FMT) for patients with ulcerative colitis (UC) is proposed as a potential approach to resolving their dysbiosis; however, its safety and efficacy have not been confirmed. This single-arm, open-label, non-randomized study aimed to evaluate the safety and efficacy of FMT for Japanese patients with UC as the first registered clinical trial in Japan.
Methods
We enrolled 10 patients with active UC despite medical therapy. The donors were the patients' relatives and were carefully screened for infectious diseases. Fecal material was administered via colonoscopy, and the primary endpoint was the presence or absence of serious adverse events related to FMT. The secondary endpoint was a change in partial Mayo score at 12 weeks post-FMT. Scores ≤2 were considered a clinical response. Fecal samples were collected to follow changes in gut microbiota, while extracted complementary DNA were analyzed by a next-generation sequencer. We obtained written informed consent from all patients and donors. This study was approved by our Institutional Review Board and is registered in the University hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN 000012814).
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon and rectum that is induced by an inappropriate mucosal immunological response. The precise pathophysiology of UC is unclear, although this immunological dysregulation may correlate with changes in the colonic environment including the intestinal microbiota.1234 Recent studies have shown that the composition of the microbiota in patients with UC differs from that in healthy controls and that microbiota imbalance, also known as “dysbiosis,” is associated with intestinal inflammation.5 Previous reports of murine models suggested that different populations of intestinal microbiota influence one another by proliferating in an identical environment and that the microbiota are remarkably stable. Garret et al.4 showed that wild-type mice developed colitis when co-housed with T-bet-/-×RAG2-/- UC mice, a spontaneously induced colitis model. Elinav et al.6 reported the same phenomenon when co-housing wild-type mice with ASC or NLRP6 knock-out mice, which disrupted the homeostasis of the intestinal mucus layer. These results showed that intestinal microbiota can be transplanted into genetically dissimilar mice in an identical environment and led to the hypotheses that resolving dysbiosis could potentially ameliorate colitis and that human fecal microbiota transplantation (FMT) may be a method by which to accomplish this.
Several studies have shown that certain probiotics induce symbiosis in patients with UC;78 however, the meta-analysis of Mallon et al.9 suggested that probiotics do not induce remission in patients with active UC. This failure may result from the overwhelming difference in bacterial counts between probiotics and the human intestinal microbiota. To address this problem, much attention has been directed to human feces, which contain microbiota metabolites and much more varied bacteria than those contained in probiotics. Dysbiosis leads to impaired colonization resistance to microbial pathogens, while FMT is thought to induce the metabolic function and colonization resistance of the microbiota and recover the composition of the intestinal flora in patients with UC. In recurrent Clostridium difficile infections (rCDI), FMT had a much higher cure rate than standard antibiotic treatment.10 Additionally, previous reports showed that FMT might restore the intestinal microbial balance in human diseases.1112131415
Almost all of the drugs currently developed for UC therapy, including biologics and tacrolimus, are immunosuppressive. These drugs play a pivotal role in IBD therapy; however, FMT may address different aspects of UC pathophysiology by resolving dysbiosis and improving UC therapy. Much effort has been involved in determining whether FMT is effective against patients with active UC.21216 In Canada and the Netherlands, Moayyedi et al.17 reported that FMT induced remission in a significantly greater percentage of patients with active UC than did the placebo; however, Rossen et al.18 reported no statistically significant difference in clinical and endoscopic remission between UC patients who received fecal microbiota from a healthy donor and those who received their own fecal microbiota. Notably, recent reports showed that the gut microbiome in the Japanese population is considerably different from those of other populations.1920 Thus, the authors suggest that there may be differences in the effectiveness of FMT for UC patients in Japan compared with that in other populations. A remaining concern is an open research problem regarding the safety and efficacy of FMT. Here we performed a single-arm, open-label, non-randomized study of the safety and efficacy of FMT. To our knowledge, this is the first registered study of FMT in UC in Japan and could help ensure the availability of FMT in Japanese patients with gastrointestinal disorders.
The ethics committee at Keio University School of Medicine approved the protocol (#20130383), and all participants provided written informed consent. The study was registered at the University hospital Medical Information Network (UMIN) Center (UMIN 000012814).
In this single-center, open-label, non-randomized study, the safety and efficacy of FMT was evaluated in patients with moderate-to-severe active UC. Clinical follow-up was performed 12 weeks post-FMT. The primary endpoint of the study was at week 12.
Eligible patients were aged ≥15 years with active UC defined as a Mayo Clinic score ≥4 with endoscopic Mayo Clinic score ≥1 despite treatment with corticosteroids, immunomodulators, tacrolimus, and/or anti-tumor necrosis factor agents. Concomitant treatments for UC, such as mesalamine, immunosuppressive therapy (e.g., azathioprine), or anti-tumor necrosis factor agents were permitted. Patients were excluded if their disease severity required hospitalization or if they were pregnant or unable to give informed consent.
Healthy relatives within the second-degree relationship (≥20 years of age) were screened using stool and serology screening for bacterial, parasitic, and viral pathogens. A complete overview of the donor screening process is shown in Table 1.
As we previously reported,21 the donors were instructed to collect a fecal sample in an AneroPack™ (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) and bring the pack to the hospital at 4℃ on the day of the scheduled FMT. Approximately 50 to 300 g of feces was collected from donors, dissolved in 50 to 100 mL of saline, and filtered through a metal strainer to make a liquid slurry. Fecal materials were administered to the patient within 6 hours after collection by the donor via colonoscopy following standard bowel preparation (2 L polyethylene glycol solution).
The primary endpoint of the study was the presence or absence of serious adverse events related to FMT. The secondary endpoint was a change in the partial Mayo (pMayo) score 12 weeks after FMT. Scores ≤2 were considered a clinical response
Fecal samples were longitudinally collected from patients at weeks 0, 1, 2, 4, 8, and 12 post-FMT and from donors on the day of the FMT. In total, 58 fecal samples were collected from 10 patients, and the collected fresh feces were stored under anaerobic conditions in an AneroPack™ (Mitsubishi Gas Chemical Co., Inc.) at 4℃. Within 24 hours after sampling, the feces were frozen in 20% glycerol (Wako Pure Chemical Industries Ltd., Osaka, Japan)/phosphate-buffered saline solution (Life Technologies, Tokyo, Japan) by liquid nitrogen and stored at −80℃ until use.
Bacterial DNA were isolated as described previously.19 Briefly, bacterial DNA was isolated by the enzymatic lysis method using lysozyme (Sigma-Aldrich Co., LCC., Tokyo, Japan) and achromopeptidase (Wako Pure Chemical Industries Ltd.). DNA samples were then purified by treatment with ribonuclease A (Wako Pure Chemical Industries Ltd.), followed by precipitation with 20% polyethylene glycol solution (PEG6000 in 2.5 M sodium chloride). The DNA was then pelleted by centrifugation, rinsed with 75% ethanol, and dissolved in tris-EDTA buffer.
The fecal DNA samples were sequenced using the 454 GS FLX Titanium and FLX+ (Roche, Basel, Switzerland) sequencing system. The detailed protocols were described previously.19 Briefly, the 16S rRNA gene V1 to V2 region was amplified by PCR by using the primers 27Fmod and 338R containing 454 primer sequences A and B and a unique 10-bp barcode sequence in 27Fmod. The PCR amplicons were sequenced to obtain reads, and the reads with an average quality value <25, mismatches to both universal primers, and possible chimeric reads were removed. Among the high-quality reads, 3,000 reads per sample were randomly selected and grouped into operational taxonomic units (OTUs) by clustering using the UCLUST algorithm with a 96% identity threshold. Taxonomic assignments for each OTU were made by similarity search against the public 16S and National Center for Biotechnology Information (NCBI) genome databases using the GLSEARCH program. For assignment at the phylum, family, genus, and species levels, sequence similarity thresholds of 70%, 90%, 94%, and 96%, respectively, were employed. All of the high-quality 16S V1 to V2 sequences analyzed in this study were deposited into the DNA Data-Bank of Japan (DDBJ)/GenBank/ European Molecular Biology Laboratory (EMBL) database under accession number DRA004886. UniFrac distance and principal coordinate analysis were used to assess the similarity of the microbiota structure of each pair of samples.22
Results are expressed as mean±SEM. Groups of data were compared using Student t-test. For multiple comparisons, the statistical analysis was performed using the Kruskal-Wallis one-way ANOVA test and the Tukey-Kramer test. Differences were considered statistically significant when the P-value was <0.05. All of the analyses were conducted using GraphPad Prism Software version 6 (GraphPad Software, San Diego, CA, USA).
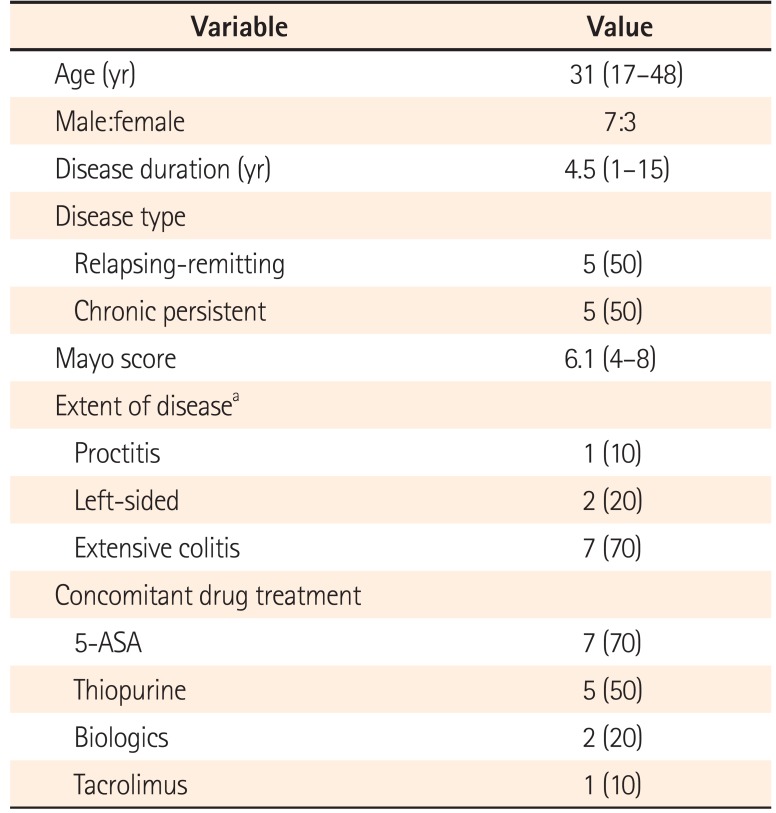
We recruited 10 patients from March 2014 through October 2015, including three with mild disease and seven with moderate disease. Patients' baseline and clinical characteristics are shown in Table 2. All patients had a history of immunosuppressive treatment, while six were naïve to biologics treatment.
Screening of 12 healthy subjects for stool pathogens and serology resulted in 10 eligible donors; two were excluded because of positive screening results for infectious agents in their feces. Donors comprised two spouses, four parents, and four sisters. All donors donated feces that were used for patient infusion. The donors' mean age was 45 years (range, 31–66 years), of which four were male.
Previous studies reported serious adverse events following FMT, including small bowel perforation, cytomegalovirus infection, and carcinoma. In our study, two patients required additional therapy during the 12 weeks following FMT; however, no severe FMT-associated adverse event was observed except for exacerbation of the UC itself. Six patients showed exacerbation of colitis and three showed amelioration of colitis. Only one patient (“J” in Fig. 1A) showed a clinical response (pMayo score pre-FMT, 4; post-FMT, 1). Two other patients (“D” and “E” in Fig. 1A) did not satisfy the definition of clinical response. Overall, no significant difference was found between the pre-FMT and post-FMT pMayo scores (Fig. 1). These results suggested that our single FMT protocol was safe; however, it had limited effectiveness for active UC.
Intestinal microbial profiling was conducted by extracting genomic DNA from the patient and donor fecal samples using the protocol described above. Fifty-nine samples were collected from the 10 patients and 10 samples were collected from the 10 donors.
Comparison of the donor and patient samples at baseline revealed that the diversity index of the fecal microbiota in the healthy donors showed higher diversity than that of the patients, although there was no significant difference (P=0.11). The diversity of the microbiota in the patients' samples increased slightly at the 12-week post-FMT evaluation; however, the findings were not significant (P=0.29) (Fig. 2A).
The taxonomic profiles showed that the phylum Firmicutes was dominant in almost all of the donors and patients with no significant difference among them. The taxonomic profiles of the patient samples showed no significant change pre-FMT versus post-FMT (Fig. 2B, Supplementary Fig. 1). Interestingly, the abundance of Bifidobacterium longum was restored in patients with active UC following FMT; however, there was no significant difference pre-FMT versus post-FMT (P=0.08) (Fig. 2C, Supplementary Fig. 2). Redundancy analysis showed that the microbiota composition of donors overlapped with that of patients at baseline and at 12 weeks post-FMT (Supplementary Fig. 3). These results indicate that the single FMT via colonoscopy for patients with UC was conducted safe; however, it had limited clinical effectiveness and potential to change the intestinal microbiota.
To our knowledge, this is the first registered trial to evaluate the safety and efficacy of FMT in Japan. Our results suggest that FMT for patients with UC was safe; however, we failed to show its clinical efficacy. The limitation of our study design was the sample size; however, the ethics committee at our institution asked us to prioritize the safety evaluation over the efficacy evaluation. Thus, this being the first registered trial in Japan, we concluded the including >10 patients would be unethical.
Analysis of intestinal microbiota showed that the abundance of B. longum tended to be lower in UC patients than in healthy subjects. Our results showed a trend toward an increase in B. longum after FMT; however, whether FMT induced symbiosis in UC patients is unclear.
A previous report showed that B. longum altered gut luminal biotin and butyrate metabolism by modifying the gut microbial community.23 Short-chain fatty acids (SCFAs) including butyrate are produced by the fermentation of dietary fiber by intestinal microbiota, and several reports have shown that SCFAs could induce gut homeostasis.2425 These findings suggest that FMT can change the gut microbial community by altering SCFA metabolism. We failed to show a relationship between an abundance of B. longum and clinical improvement following FMT; however, previous reports showed that B. longum alleviated experimental colitis in murine models.262728 Additionally, Tamaki et al.29 concluded that supplementation with B. longum was well tolerated and reduced the UC disease activity index.
In this study, we administered donor feces only once, reflecting our primary endpoint of evaluating FMT safety. Moayyedi et al.17 reported that FMT could induce remission in patients with active UC; however, the authors gave retention enemas to patients once per week for 6 weeks. Therefore, the frequency of our FMT protocol may be a major limitation for inducing remission in active UC patients. In addition to FMT frequency, donor selection is a critical issue. We selected donors from among patients' relatives, although Moayyedi et al.17 stated that most FMT successes were related to the use of unrelated donor specimens. Previous reports showed that the intestinal microbiota reflect one's living environment and diet,30 indicating that relatives may have similar intestinal microbiota. A varied microbiota is vital to the induction of symbiosis in patients with UC; therefore, the ideal donors in future FMT studies would be healthy volunteers unrelated to the patient.
Further evaluations are needed to elucidate the efficacy of FMT for UC; however, this form of clinical development with high ethical concerns should be very fairly and carefully performed until its safety is confirmed. Nevertheless, FMT for patients with UC seems to have positively received much attention in recent years despite the lack of definite evidence due to the influence of the great success of rCDI. Thus, we believe that the current negative study plays a pivotal role in cautioning researchers to carefully pursue the clinical development of FMT for different diseases, nations, and protocols.
ACKNOWLEDGEMENTS
The authors thank A. Hayashi and M. Mutaguchi (Keio University) for their technical assistance and helpful discussions. The authors also thank Y. Nakazato, R. Bessho, H. Ogata, and Y. Iwao (Keio University) for their helpful discussions.
Notes
References
1. Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016; 14:127–138. PMID: 27175113.

2. Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012; 107:1452–1459. PMID: 23034604.

3. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010; 28:573–621. PMID: 20192811.

4. Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007; 131:33–45. PMID: 17923086.

5. Takaishi H, Matsuki T, Nakazawa A, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008; 298:463–472. PMID: 17897884.

6. Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011; 145:745–757. PMID: 21565393.

7. Chen WX, Ren LH, Shi RH. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J Gastroenterol. 2014; 20:15657–15663. PMID: 25400449.

8. Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006; 24(Suppl 3):90–95. PMID: 16961752.

9. Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007; (4):CD005573. DOI: 10.1002/14651858.CD005573.pub2. PMID: 17943867.

10. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368:407–415. PMID: 23323867.

11. Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013; 108:1620–1630. PMID: 24060759.

12. Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013; 19:2155–2165. PMID: 23899544.

13. Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol. 2013; 27:127–137. PMID: 23768558.

14. Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014; 5:e00893–e00914. DOI: 10.1128/mBio.00893-14. PMID: 24939885.

15. Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014; 306:G310–G319. PMID: 24284963.
16. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014; 8:1569–1581. PMID: 25223604.

17. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.e6. PMID: 25857665.

18. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118.e4. PMID: 25836986.

19. Nishijima S, Suda W, Oshima K, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016; 23:125–133. PMID: 26951067.

20. Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007; 14:169–181. PMID: 17916580.

21. Matsuoka K, Mizuno S, Hayashi A, Hisamatsu T, Naganuma M, Kanai T. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014; 63:69–74. PMID: 25500625.

22. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011; 5:169–172. PMID: 20827291.

23. Sugahara H, Odamaki T, Fukuda S, et al. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep. 2015; 5:13548. DOI: 10.1038/srep13548. PMID: 26315217.

24. Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013; 145:396–406.e10. PMID: 23665276.

25. Masui R, Sasaki M, Funaki Y, et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis. 2013; 19:2848–2856. PMID: 24141712.

26. Miyauchi E, Ogita T, Miyamoto J, et al. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PLoS One. 2013; 8:e79735. DOI: 10.1371/journal.pone.0079735. PMID: 24255712.

27. Srutkova D, Schwarzer M, Hudcovic T, et al. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute DSS-induced colitis in strictly strain-specific manner. PLoS One. 2015; 10:e0134050. DOI: 10.1371/journal.pone.0134050. PMID: 26218526.

28. Wei P, Yang Y, Ding Q, et al. Oral delivery of Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to combat ulcerative colitis. J Med Microbiol. 2016; 65:160–168. PMID: 26567174.

29. Tamaki H, Nakase H, Inoue S, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016; 28:67–74. PMID: 26418574.

30. Nakayama J, Watanabe K, Jiang J, et al. Diversity in gut bacterial community of school-age children in Asia. Sci Rep. 2015; 5:8397. PMID: 25703686.

Supplementary Material
Supplementary Fig. 1
Taxonomic profiles. Each donor and each patient pre- (0 week), and 1, 2, 4, and 12 weeks post-fecal microbiota transplantation.
Supplementary Fig. 2
Abundance of Bifidobacterium longum in donors and patients pre- and 12 weeks post-fecal microbiota transplantation. OUT, operational taxonomic unit.
Supplementary Fig. 3
Redundancy analysis of microbiota from donors and patients pre- and 12 weeks post-fecal microbiota transplantation.
Fig. 1
Change in partial Mayo (pMayo) score. (A) Change in pMayo score in each patient, and (B) comparison of pMayo score pre- and 12 weeks post-fecal microbiota transplantation.
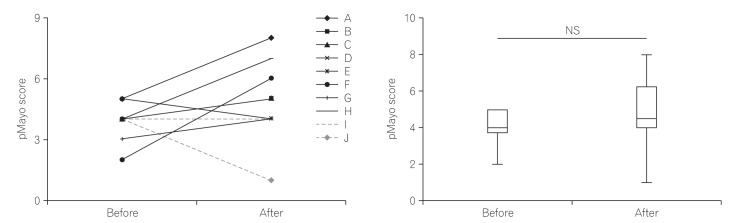
Fig. 2
Analysis of the microbiome. (A) Microbiota diversity (Shannon index) of donors and patients pre- and 12 weeks post-fecal microbiota transplantation (FMT). (B) Comparison of the top four fecal bacteria at the phylum level, and (C) the top five fecal bacteria at the species level comparing pre- and post-FMT. The average operational taxonomic unit abundance is shown for each group.
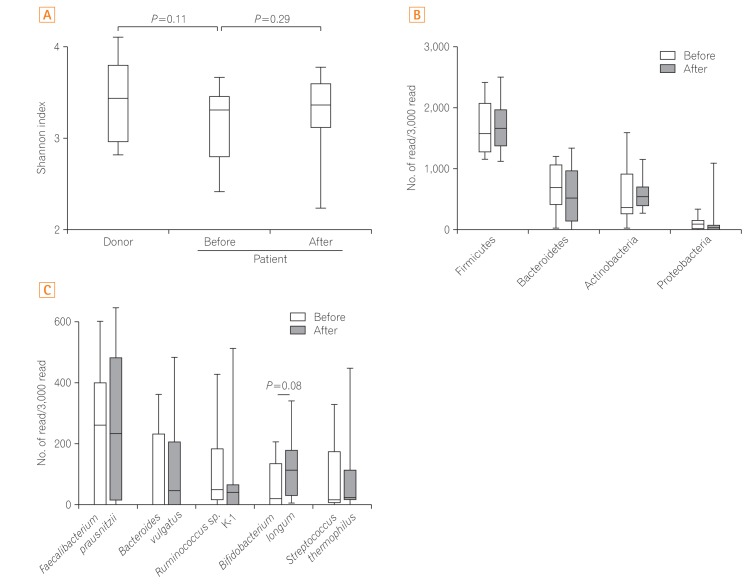
Table 1
Overview of Donor Screening Process
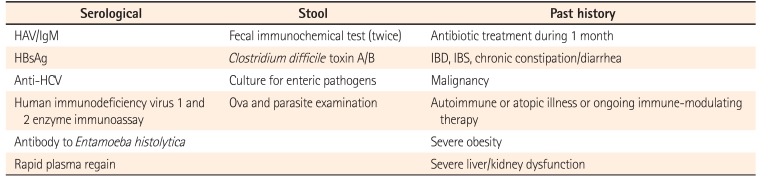
Table 2
Patients' Baseline Characteristics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download