Abstract
Clinical features of masticator-space abscess (MSA) are very similar to those of parotitis or temporomandibular disorder (TMD), making early differential diagnosis difficult. Local causes of MSA include nerve block anesthesia, infection after tooth extraction, and trauma to the temporomandibular joint (TMJ); the systemic cause is immunodeficiency. Odontogenic causes account for most etiologies, but there are also unusual causes of MSA. A 66-year-old male patient visited the emergency room (ER) presenting with left-side TMJ pain three days after receiving an acupressure massage. He was tentatively diagnosed with conventional post-trauma TMD and discharged with medication. However, the patient returned to the ER with increased pain. At this time, his TMD diagnosis was confirmed. He made a third visit to the ER during which facial computed tomographic (CT) images were taken. CT readings identified an abscess or hematoma in the left masticator space. After hospitalizing the patient, needle aspiration confirmed pus in the infratemporal and temporal fossa. Antibiotics were administered, and the abscess was drained through an incision made by the attending physician. The patient's symptoms decreased, and he was discharged.
The masticator space is defined by the superficial layer of the deep cervical fascia and is composed of a suprazygomatic (temporal fossa) portion and a deep nasopharyngeal (infratemporal fossa) portion. The suprazygomatic portion in the temporal fossa contains the temporal muscle. The infrazygomatic portion is separated by the mandibular ramus into medial and lateral parts. The medial part includes the medial pterygoid muscle; the lateral part contains the masseter muscle, which communicates with the lateral pterygoid muscle1,2. Abscess formation in the masticator space is primarily caused by spread of odontogenic infection. Similar to temporomandibular disorder (TMD), the initial symptoms are trismus and temporomandibular joint (TMJ) pain. Orofacial pain and limitation of jaw movement without obvious odontogenic infection commonly lead to the diagnosis of TMD. For an accurate early diagnosis of unusual infection in the masticator space, clinicians need to be aware of case reports of masticator-space abscess (MSA) without odontogenic origins. This report describes a case of abscess formation in the masticator space after an acupressure massage.
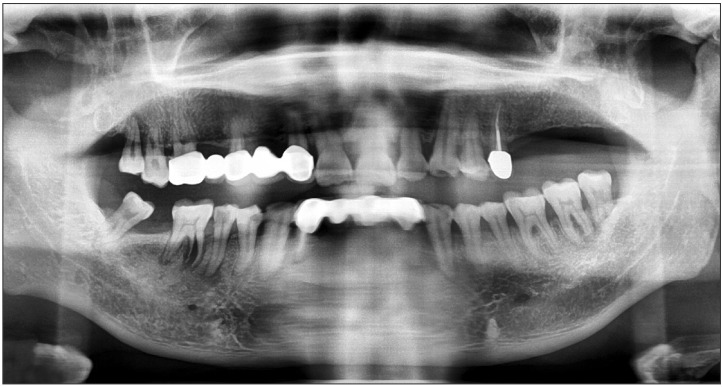
A 66-year-old male visited the emergency room (ER) complaining of acute pain in the left cheek and pre-auricular area three days after receiving a facial acupressure massage. He had tenderness in the left masseter and pain when clenching his teeth, opening his mouth, and moving his mouth laterally. There were no specific abnormal radiographic findings in panorama view (Fig. 1) or TMJ tomography.(Fig. 2) He had no history of systemic symptoms or fever. During his first visit to the ER, he was tentatively diagnosed with TMD. He was discharged with medication and informed to remain cautious for serious TMD symptoms. After five days, he returned to the ER because his pain increased and expanded beyond the left TMJ area, decreasing his ability to open his mouth. During his second visit, he was again diagnosed with TMD and discharged with an intravenous analgesic. Two days after his second visit to the ER, he presented with severe pain and trismus. At this time, his maximum mouth opening distance had decreased to 25 mm. He also had tenderness in his left masseter, temporalis, and pre-auricular area. We observed no swelling in his left cheek. Contrast-enhanced facial computed tomography (CT) was performed to identify the pain source. The CT scan illustrated a 4.2×1.9×2.3 cm collection of lobulated fluid with peripheral enhancement; we suspected an abscess or an organized hematoma in the left masticator space.(Fig. 3) The patient was admitted to the hospital and administered empirical antibiotics, including intravenous cefoperazone/sulbactam, metronidazole, and aminoglycoside. Needle aspiration was performed to check for an abscess or hematoma in the masticator space and confirmed pus. The culture of this pus showed Streptococcus intermedius.(Fig. 4) At admission, the following laboratory results were obtained; erythrocyte sedimentation rate, 59 mm/hour; C-reactive protein, 3.1 mg/dL; and white blood cell count, 11,390/µL. These results led to a final diagnosis of MSA. Intra-oral incision and drainage were performed under general anesthesia. Additionally, extra-oral incision and drainage were performed to access the temporal space. After these treatments, his symptoms subsided, and he was discharged without any clinical symptoms. The final CT scan before discharge revealed that the peripheral enhancing fluid collection in the left masticator space had decreased in size.(Fig. 5) He continued to show improvement and had no further symptoms.
The masticator space lies deep within the skull and consists of a suprazygomatic portion and a nasopharyngeal portion. The space contains four masticator muscles: the temporalis, the masseter, and the lateral and medial pterygoids. Abscess formation in the masticator space is relatively rare, and odontogenic causes account for most MSAs. Odontogenic causes include periodontitis, pericoronitis, dental caries, suppurative pulpitis, and post-tooth extraction infection3,4. In addition to these common causes, sinusitis5, sinus fracture6, posttraumatic osteomyelitis of the maxilla7, temporomandibular arthroscopy8, and trauma3,9 can also cause MSA4,10.
Typical clinical symptoms of MSA include trismus and tenderness in the masticator muscles; these symptoms are similar to those of TMD. These symptoms, in the absence of clear odontogenic infection, might lead to a diagnosis of TMD. Matsumura et al.11 reported a patient with a peri-temporomandibular abscess that was a complication of acupuncture treatment and was tentatively diagnosed as TMJ arthritis. Hasegawa et al.3 also reported two such cases. First, a patient was diagnosed with TMD after extraction of an upper second molar due to severe periodontitis. Second, a patient who had class IV caries in the lower left second molar and pericoronitis in the lower left third molar tooth was first diagnosed with TMD. Both patients were ultimately diagnosed with MSA. Kim et al.9 reported three cases of MSA that did not have an odontogenic origin. Of these, one patient had no specific etiology, one had an upper respiratory infection, and the other had experienced trauma to the chin. These cases demonstrate the difficulty of differential diagnosis between MSA and TMD, especially when there is no clear etiology.
The patient in the present report complained of trismus and facial pain after receiving an acupressure massage with particularly strong pressure. He had not received dental treatment prior to the massage. At his first ER visit, he was afebrile and had no swelling on either TMJ area. His clinical symptoms and history suggested TMD caused by trauma. MSA was only confirmed after facial-bone CT scan and needle aspiration. No clear primary infection source was identified. The analogical cause of this case was a closed trauma, which is rarely reported in the literature. Goldschmidt et al.12 described a mechanism by which closed trauma can increase the chance of TMJ infection.
Microorganisms can invade an infection site via three inoculation routes: direct, contiguous, or hematogenous13. Direct inoculation includes acupuncture, needle injection, and dental extraction. Contiguous inoculation originates from adjacent inflammatory structures to the focal site. Finally, hematogenous inoculation involves intravenous seeding of microorganisms from a distant primary infectious site9; hematogenous inoculation is rare in MSA cases14. Direct inoculation, such as post-extraction complications, is common among MSA cases. Closed injury increases the risk of secondary infection through a hematogenous route; however, the mechanism for this is unclear. There are three possible etiological explanations for TMJ infection: 1) hyperemia increases the chance of exposure to microorganisms; 2) damage to local anatomy allows bacteria easy access to the focal site; and 3) hematoma formed by trauma provides a local culture medium for bacterial growth15.
The present patient had no direct inoculation history around the masticator space. CT scan and clinical findings also showed no inflammation in the tissue around the masticator space. There were no systemic predisposing factors, but there were potential local infection sources development of MSA. A panoramic radiography of the infection site showed a fractured root in the right lower first molar with an infectious state. The patient had no signs of intra-oral chronic periodontitis. Pus culture results from the abscess offered another clue for infection source and route.
S. intermedius, a member of the Streptococcus milleri group, was detected in the cultured pus sample. The S. milleri group constitutes normal flora of the mouth that have the ability to cause suppurative infection16. Of the S. milleri group members, S. intermedius is frequently isolated from dental plaque17 and is most likely to be associated with head and neck infection18. Another study found that abscesses caused by S. intermedius infection are associated with hematogenous spread and deep-tissue infection19. Based on these findings, it is possible that a blunt trauma to the head caused the hematoma within the masticator space, allowing S. intermedius to spread hematogenously from an intra-oral primary infection source. However, the exact source or route of the infection was not confirmed, and more research is needed.
As with other facial abscess, antibiotic therapy combined with surgical drainage successfully resolved the MSA. To obtain optimum results, appropriate antibiotic selection based on culture sensitivity and a specific early diagnosis via CT or magnetic resonance imaging are needed.
Most MSAs originate from an odontogenic infection. Typical symptoms of MSAs, which are similar to those of TMD, include trismus and pain in the masticator muscles. The clinical symptoms might mislead clinicians to a wrong diagnosis, especially when the etiology is unusual. Delayed treatment of MSA increases the infection extent and severity. To prevent this, clinicians must bear in mind that rarely reported etiologies, like facial massage, are capable of initiating an infection.
References
1. Curtin HD. Separation of the masticator space from the parapharyngeal space. Radiology. 1987; 163:195–204. PMID: 3823435.

2. Chong VF, Fan YF. Pictorial review: radiology of the masticator space. Clin Radiol. 1996; 51:457–465. PMID: 8689819.

3. Hasegawa T, Shibuya Y, Kuroki S, Takeuchi J, Yokoo S, Umeda M, et al. Two cases of masticator space abscess initially diagnosed as temporomandibular joint disorder. Kobe J Med Sci. 2008; 54:E163–E168. PMID: 19246964.
4. Kamath MP, Bhojwani KM, Mahale A, Meyyappan H, Abhijit K. Infratemporal fossa abscess: a diagnostic dilemma. Ear Nose Throat J. 2009; 88:E23. PMID: 19444778.
5. Raghava N, Evans K, Basu S. Infratemporal fossa abscess: complication of maxillary sinusitis. J Laryngol Otol. 2004; 118:377–378. PMID: 15165316.

6. Weiss BR. Infratemporal fossa abscess unusual complication of maxillary sinus fracture. Laryngoscope. 1977; 87:1130–1133. PMID: 875575.

7. Connor SE, Davitt SM. Masticator space masses and pseudomasses. Clin Radiol. 2004; 59:237–245. PMID: 15037135.

8. Chossegros C, Cheynet F, Conrath J. Infratemporal space infection after temporomandibular arthroscopy: an unusual complication. J Oral Maxillofac Surg. 1995; 53:949–951. PMID: 7629629.
9. Kim HM, Kim TW, Hwang JH, Lee DJ, Park NR, Song SI. Infection of the temporomandibular joint: a report of three cases. J Korean Assoc Oral Maxillofac Surg. 2011; 37:510–514.

10. Morrison A, Brady J. Temporal space abscess secondary to mandibular dental extraction. Oral Health. 2009; 99:17–21.
11. Matsumura Y, Inui M, Tagawa T. Peritemporomandibular abscess as a complication of acupuncture: a case report. J Oral Maxillofac Surg. 1998; 56:495–496. PMID: 9541352.

12. Goldschmidt MJ, Butterfield KJ, Goracy ES, Goldberg MH. Streptococcal infection of the temporomandibular joint of hematogenous origin: a case report and contemporary therapy. J Oral Maxillofac Surg. 2002; 60:1347–1353. PMID: 12420272.

13. Bounds GA, Hopkins R, Sugar A. Septic arthritis of the temporomandibular joint--a problematic diagnosis. Br J Oral Maxillofac Surg. 1987; 25:61–67. PMID: 2948546.

14. Hardin CW, Harnsberger HR, Osborn AG, Doxey GP, Davis RK, Nyberg DA. Infection and tumor of the masticator space: CT evaluation. Radiology. 1985; 157:413–417. PMID: 4048449.

15. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991; 22:503–514. PMID: 1852426.

16. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988; 10:257–285. PMID: 3287560.

17. Whiley RA, Fraser H, Hardie JM, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the "Streptococcus milleri group". J Clin Microbiol. 1990; 28:1497–1501. PMID: 2380375.

18. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serologic characteristics. Am J Clin Pathol. 1995; 104:547–553. PMID: 7572815.

19. Claridge JE 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus ("Streptococcus milleri group") are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001; 32:1511–1515. PMID: 11317256.
Fig. 2
Temporomandibular joint tomography view shows limited mouth opening. A, D. Temporomandibular joint tomography when the mouth is closed. B, C. Temporomandibular joint tomography when the mouth is opened.
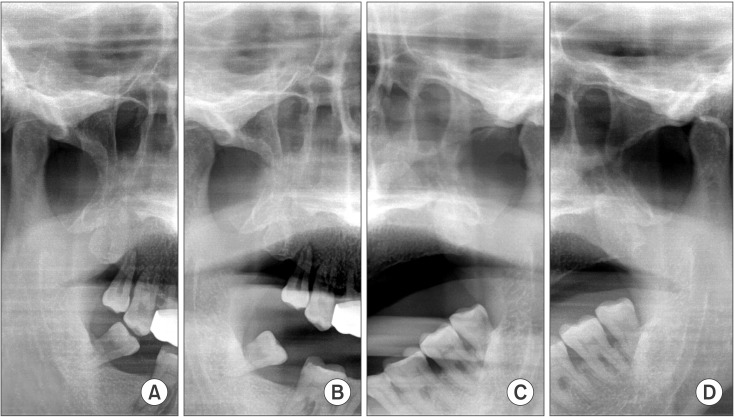
Fig. 3
Contrast-enhanced facial computed tomography shows 4.2×1.9×2.3 cm fluid collection. A. Preoperative image of computed tomography axial view. Lobulated fluid collection with thin enhancing rim is seen in left masticator space. B. Preoperative image of computed tomography coronal view with fluid collection in left masticator space.
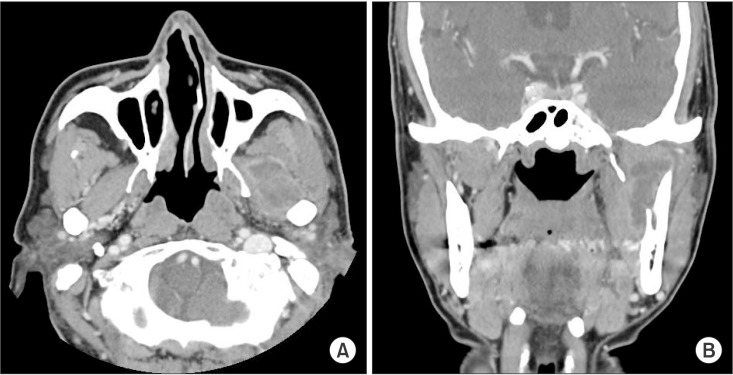
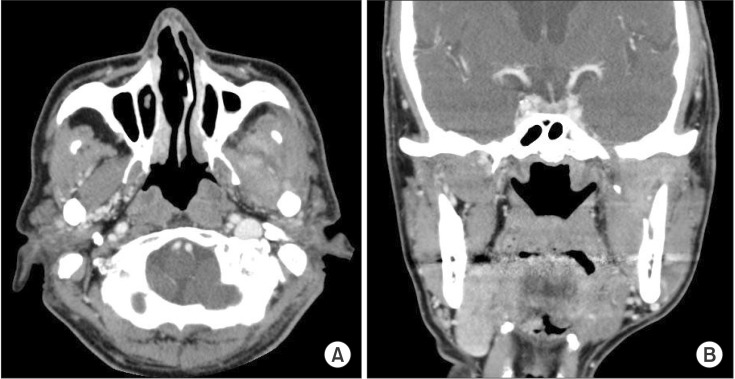
Fig. 5
Contrast-enhanced facial computed tomography shows 3.2×2.1×3.8 cm fluid collection. A. Follow-up image of computed tomography axial view. Decreased size of peripheral enhancing fluid collection is seen in left masticator space. B. Follow-up image of computed tomography coronal view with decreased fluid collection in left masticator space.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download