Abstract
Objectives
This study sought to elucidate the effect of autogenous tooth bone material by experimenting on minipig's maxillary sinus and performing histological and histomorphometric analyses.
Materials and Methods
Five 18-24 month-old male minipigs were selected, and right maxillary sinuses were grafted with bone graft material made of their respective autogenous teeth extracted eight weeks earlier. The left sides were grafted with synthetic hydroxyapatite as control groups. All minipigs were sacrificed at 12 weeks after bone graft, which was known to be 1 sigma (σ) period for pigs. Specimens were evaluated histologically under a light microscope after haematoxylin-eosin staining followed by semi-quantitative study via histomorphometric analysis. The ratio of new bone to total area was evaluated using digital software for calculation of area.
Results
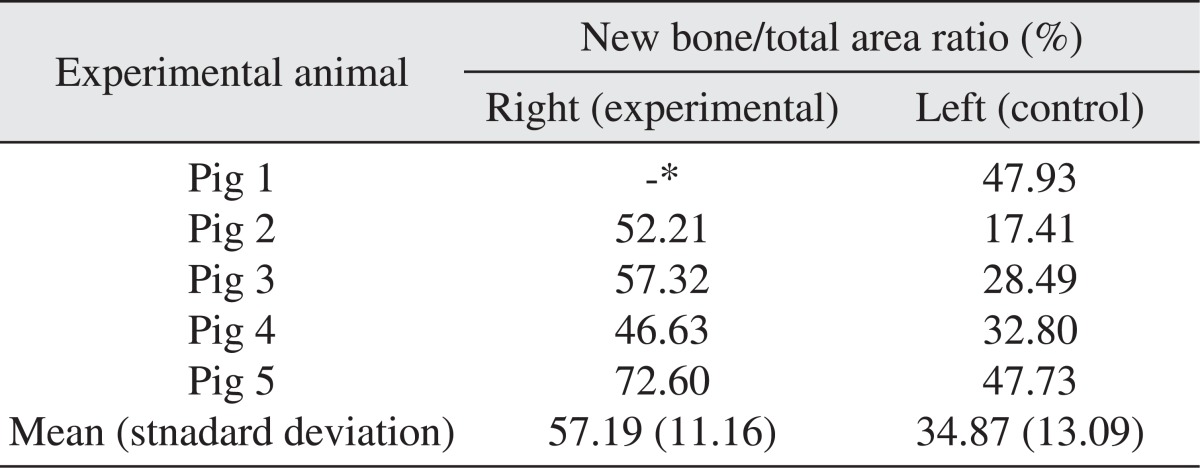
All specimens were available, except one on the right side (experimental group), which was missing during specimen preparation. This study demonstrated new bone at the periphery of the existing bone in both groups, showing evidence of bone remodeling, however, encroachment of new bone on the central part of the graft at the 1 σ period was observed only in the autogenous tooth bone group (experimental group). Histomorphometric analysis showed more new bone formation in the experimental group compared to the control group. Although the difference was not statistically significant (P>0.05), the mean percentage area for new bone for the experimental and control groups were 57.19%±11.16% and 34.07%±13.09%, respectively.
Conclusion
The novel bone graft material using autogenous tooth is a good alternative to autogenous bone, comparable to autogenous bone, and outperforming synthetic hydroxyapatite bone graft materials in terms of bone regeneration capacity. Augmentation with autogenous tooth bone materials will reduce donor site morbidity without hampering the safety of the autogenous bone graft.
Bone grafting started to be applied to the pneumatized maxillary sinus in blade implant placement in the late 1970s after grafting in the maxillary sinus was performed by Boyne with the intention of bone augmentation for the first time in 1960. In 1980, Boyne and James1 published their first paper describing the outcomes in the aforesaid case. With the introduction of root-form implants, the necessity of alveolar bone augmentation has increased; thus, the frequency of maxillary sinus bone graft has started to rise gradually. Afterward, with the advancements in surgical techniques and implant materials, it is one of the most common and successful bone graft options in the maxillofacial area at present. Many clinicians developed a variety of surgical techniques and approaches, and the outcomes of various bone grafting techniques and materials in clinical practice were reported in the 1996 Sinus Consensus Conference2.
Tricalcium phosphate was the first alterative bone substitute successfully used as maxillary sinus bone graft material3. After that, lots of bone graft materials such as allogenic grafts, synthetic grafts, and xenogenic grafts have been used individually or in combination with autogenous bone in maxillary sinus bone graft. Favorable results of retrospective studies of several bone graft materials were also reported at the 1996 Sinus Consensus Conference2. Afterward, with very stable outcomes observed when autogenous bone was employed as maxillary sinus bone graft material in the maxillary molars with at least moderate bone resorption or pneumatization, especially in severely atrophic maxilla, the use of autogenous bone grafts has been recommended. In addition, the unique advantages of autogenous bone as maxillary sinus bone graft material were demonstrated to enable their versatile and useful applications to bone grafting techniques for different defects or lesions.
Recently introduced in South Korea, autogenous tooth bone graft is attracting public attention because it is known to feature enhanced availability of autogenous grafts; thus most likely leading to higher success rates4. This graft material was developed as a result of paying attention to the fact that the composition of teeth is almost identical to that of bone, and that tooth-derived material is made of bone components harvested using extracted autogenous teeth5,6. Since it is autogenous, it is immunologically identical to self and without psychological rejection in patients. Furthermore, it is expected to have osteoinduction activity as well as osteoconduction7-9.
In this context, this study intended to clarify any effect of autogenous tooth bone grafts - made by processing extracted teeth that had been discarded in the past - on new bone formation in maxillary sinus bone grafting based on findings of experiments on miniature pigs as lab animals.
Five healthy male miniature pigs weighing approximately 40 kg and with ages of 24 months or so (Prestige World Genetics, Pyeongtaek, Korea) were used as animal subjects. In accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines, every experiment was conducted in the operating room within the laboratory, and all procedures for selection, care, and surgical treatments of animal subjects (lab animals) were implemented after obtaining IACUC's approval (Medikinetics-IACUC: 100407-001). Any and all surgical procedures and techniques were performed and applied under general anesthesia.
Atropin (Kwangmyung Pharmaceutical Ind. Co. Ltd., Seoul, Korea) was intravenously administered; xylazine (Rompun; Bayer Korea Co., Seoul, Korea) and ketamin 30 mg (Ketara; Yuhan Co., Seoul, Korea) were intramuscularly injected to induce general anesthesia; endotracheal intubation was then performed using Miller sized-4 laryngoscope and standard straight tube with inner diameter of 5.5 mm and cuff (Portex, Kent, UK); general anesthesia was maintained with 1% euflurane (Gerolan; Choongwae Pharmaceutical Co., Seoul, Korea). To prevent possible infections, epocelin (40 mg/kg body weight; Dong-A Pharm, Seoul, Korea) was intra muscularly administered both preoperatively and postoperatively; to protect the surgical site, soft food was fed to the animals until the completion of wound healing. Miniature pigs were placed in the ventral recumbency position, and then their heads were fixed followed by shaving. After disinfecting the surgical site with betadine, the site was isolated with sterile drapes. For local anesthesia, infiltration anesthesia was performed with lidocaine (2% lidocaine HCl and epinephrine, 1.8 mL; Yuhan Co., Seoul, Korea). Afterward, the left and right mandibular premolars and molars were extracted and delivered to the Korea Tooth Bank (Seoul, Korea) for processing into powdery bone graft material. After about 8 weeks, the maxillary sinus was exposed through the lateral approach, and maxillary sinus floor elevation was carried out using the outfracture osteotomy sinus graft technique.(Fig. 1) An autogenous tooth bone graft was placed on the right side for the experiment group, and hydroxyapatite (Bone Gros; BioAlpha Inc., Seongnam, Korea), on the left side for the control group. Initially included in experimental group, an animal was excluded from the experimental group because the right maxillary sinus membrane got perforated, indicating failure of maxillary sinus bone grafting. In both experimental group and control group, a bone window was prepared again in the grafted site followed by interrupted suture.
The mandibular premolars of the minipigs were extracted on both sides and prepared 8 weeks before the graft procedure. Immediately after extraction, the teeth were trimmed and cleaned of all extraneous material and sterilized in 70% alcohol. The tooth was cut in the cementoenamel junction to divide it into crown and root. Using the crown dentin block, the pulp tissue was totally removed by the retrograde technique. The teeth were crushed into powders measuring 400-800 µm under a dried and cold environment (2℃). After being washed with sterile saline at 2℃ for one hour, the teeth were immersed in 0.6N HCl (hydrochloric acid solution) at 2℃ for 30 minutes for demineralization. Afterward, the specimens were washed in distilled water for 1 hour under constant agitation to remove the acid completely. They were defatted in chloroform-methanol for 30 minutes under the same conditions as those of demineralization. After defatting, the specimens were then washed in distilled water for 3 hours under constant agitation to remove the reagents completely. Freeze-drying and sterilization were done under appropriate conditions, with the specimens stocked for graft until 8 weeks before graft.
The laboratory animals were sacrificed 12 weeks after grafting, and specimens were harvested in blocks from the grafted site along with the surrounding normal bone tissue on the floor of the maxillary sinuses. The prepared specimens were cleanly washed with normal saline, and then kept in formalin solution and sent to an independent laboratory without delay to request slide preparation and analysis. The specimens were demineralized with formic acid, and paraffin blocks were made in a routine manner. For each sample, paraffin blocks were cut into 4 µm sections, followed by hematoxylin-eosin staining and histopathological examination under an optical microscope (Olympus BX-51; Olympus Co., Tokyo, Japan). Histomorphometric analyses were performed by observing the respective slides at 100× magnification under an optical microscope. Specifically, after acquiring digital images from a digital camera mounted on the microscope, image analyzing software program (Kappa Image Base-metreo; Opto-Electronics, Gleichen, Germany) was used to determine and analyze the area of newly formed mineralized bone (NB%) in the entire lesion as seen in the acquired images.
During this study, miniature pigs remained healthy, and outcomes of the treatment in their grafted sites were good. No major complication such as infection, edema, or exposed bone graft material was observed in any group.
Typical histological findings for the experimental group are presented in Figs. 2-4. Fig. 2 shows the experimental specimens under magnification of ×100. Grafted tooth-derived graft materials (asterisks) were evident, showing multiple linear streaks as a dentinal tubular structure. New bone (arrowheads) was growing at the periphery of the tooth-derived materials in Fig. 2. Other specimen under magnification of ×400 showed bone remodeling activity as evidenced by the multinucleated giant cells of osteoclastic activity at the periphery of the graft material with multiple linear streaks (arrowhead in Fig. 3). Another experimental specimen under magnification of ×100 is shown in Fig. 4. Encroaching on the tooth-derived graft material (asterisk), new bone formation (arrowhead) was identified, showing bone remodeling as evidenced by the incremental lines. In Fig. 5, synthetic hydroxyapatite (asterisk) was partially evident in the histological section of the control group. New bone was also found at the periphery of the grafted hydroxyapatite (arrowheads) but not in the inner portion of the graft space (Fig. 5).
The area of new bone formation (B), total examined area (T), and ratio of new bone formation (B/T) were presented in Table 1. The area was measured in µm2 for the area of new bone and total area, and the ratio of new bone formation was calculated in unit %. Although there was no statistical significant difference between the two groups because of the small number of experimental animals (P>0.05), the mean values and standard deviation of the ratio of new bone formation were calculated as 57.19%±11.16% for the experimental group and 34.07%±13.09% for the control group as summarized in Table 2.
Initial immobilization10 is one of the most critically important factors for the success of dental implantation and is affected mainly by bone quality11 and bone quantity12,13 at the site of implant placement. If it is impossible to improve bone quality, bone quantity should be maximized as much as possible, especially in the case wherein there is insufficient bone quantity in the maxillary sinus floor due to sinus pneumatization in the region with low bone strength relative to that of the mandibular and maxillary molars. In this case, maxillary sinus bone grafting to fill with bone graft is essential for the success of implantation.
For maxillary sinus bone graft, autogenous bone has several advantages. In particular, spongy bone contains diverse cells that will contribute significantly to new bone growth after differentiation; autogenous bone graft materials contain bone morphogenic proteins (BMPs) that induce osteogenic cells from the surrounding tissue14. Autogenous bone also includes other growth factors involved in the healing and incorporation of the grafted bone. Cortical bone provides osteoconductive scaffolds, and it is substituted with new bone through creeping substitution1. Since autogenous bone graft has osteogenesis, osteoconduction, and osteoinduction activities without immune rejection, which leads to rapid healing, autogenous bone grafting is undoubtedly ideal for the reconstruction of defective hard tissue.
Note, however, that it has the disadvantages of limited area or quantity of harvesting, secondary defects caused in the donor site, and relatively high possibility of maxillary sinus repneumatization attributed to the resorption of the graft material over time. Hatano et al.15 argued that, to avoid possible events of repneumatization for the first two or three years after grafting, non-absorbable or slowly absorbable graft materials should be used. Since defects in the maxillary sinus are a form of "contained-type defects" with their own bony housing, most of the biocompatible bone graft materials can yield satisfactory outcomes. Recently, various graft materials used for maxillary sinus bone grafting have been shown to have no statistically significant difference in the aspects of healing; autogenous bone, allogenic bone, xenogenic bone, and synthetic bone are known to be safe. Therefore, an appropriate material can be selected depending on clinicians' preferences.
Meanwhile, the use of either xenogenic bone or synthetic bone in combination with a proper volume of autogenous bone in grafting is known to enable healing by osteogenesis and osteoinduction and shorten remarkably the length of time required for healing16. In other words, the combination of autogenous bone and bone substitute for grafting may be characterized by increased resistance to the resorption of graft material; hence the ability to maintain a sustainable volume of the material at the defective site. Since autogenous tooth bone graft materials are of self-origin, the positive effects of osteoconduction as well as osteoinduction can be expected, there is no risk of infection, it enables us to treat useful teeth that had been discarded as waste in the past, and it is relatively simple to prepare the grafts. Furthermore, minerals and organic matters can be considerably preserved in the dentin and cementum of teeth, and these preserved organic matters contain several bone growth factors including type I collagen and BMP that facilitate alveolar bone regeneration and osteogenesis4.
Lee17 devised a surgical technique capable of creating bone windows without any complication in the maxillary sinus where there are anatomical and pathological limitations as described above. After that, Song et al.18 also reported a 97.2% survival rate in outfracture osteotomy with sinus graft technique in their study. Similarly, in this study, outfracture osteotomy sinus graft technique was applied considering possible anatomical variations and relatively narrow sinus cavity volume in miniature pigs compared to humans when maxillary sinus bone grafting is performed.
Since dentin was demonstrated to contain BMPs19,20, studies on autogenous tooth bone graft materials began with the intention of evaluating osteogenesis activity through demineralized dentin matrix experiments. Butler et al.21 and Conover and Urist22 successfully extracted BMPs from the demineralized dentin matrix in rabbits. Later, McKee et al.23 studied diverse stem cells and bone grafting in miniature pigs.
Given their high growth speed and heavy weight after growth, pigs have been known to be inappropriate for study of the dental field. Note, however, that these problems were solved by the development of minipigs24, opening a new chapter of dental studies using pigs. Since porcine bone is very similar to that of human in terms of components, it is more useful for animal testing of preclinical phases. Compared to human bone, the bony trabecular of porcine bone is denser. Note, however, that the structure of the lamellar bone and bone remodeling are similar to those in human. Moreover, since the bone regeneration of pig is similar to that of human rather than that of dog25, pigs are more useful in realizing conditions in human compared to other experimental animals. In this regard, minipigs were used in this study as experimental animal. Likewise, in this study, miniature pigs characterized by similar tooth composition and alveolar bone to those of humans and frequently used in various bone grafting experiments were used as subjects so that similar conditions to those given in an in vivo experiment can be set to the maximum.
This study had some limitations, i.e., too small sample size resulting in lack of significance in statistical analysis, failure to take into account the anatomic variations at the maxillary sinus in miniature pigs, failure to analyze systematically teeth composition including enamel and dentin, and absence of long-term follow-up report. Nevertheless, relatively better remodeling was observed in the experimental group compared to the control group; the implanted autogenous tooth graft material was substituted with new bone, and newly formed bone showed marginal scalloping around the remaining tooth graft material. Based on these studies, autogenous tooth bone graft materials have been produced, with a recent study demonstrating that autogenous tooth bone graft materials have excellent osteoconduction and bone remodeling abilities because they contain minerals such as low-crystallinity hydroxylapatite and tricalcium phosphate26.
Among the currently available various bone graft materials, autogenous tooth bone graft material not only has similar bone formation capacity to the expected capacity of autogenous bone; it can also circumvent the donor site morbidity of autogenous bone graft. For this reason, many studies on the material are ongoing. The results of the experiments in this study suggest that there was no statistically significant difference; overall, however, better new bone formation was observed in the experimental group than in the control group. With no donor site morbidity occurring during the harvesting of autogenous bone and few complications such as pain or infection and similar osteoinduction and osteoconduction activities to those of autogenous bone, it can be said to be a promising material alternative to conventional autogenous bone graft materials.
Acknowledgements
The authors would like to thank Dr. In Woong Um, Chief Technical Organizer of Korea Tooth Bank, for assisting in the preparation of the material.
References
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–616. PMID: 6993637.
2. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants. 1998; 13(Suppl):11–45. PMID: 9715571.
3. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986; 30:207–229. PMID: 3516738.
4. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503. PMID: 20060336.

5. Min BM. Oral biochemistry. 1st ed. Seoul: Daehan Narae Publishing;2007.
6. Orban BJ, Bhaskar SN. Orban's oral histology and embryology. 9th ed. St. Louis: Mosby;1980.
7. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967; 12:999–1008. PMID: 4226721.

8. Kim SG, Yeo HH, Kim YK. Grafting of large defects of the jaws with a particulate dentin-plaster of paris combination. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:22–25. PMID: 10442940.
9. Park SS, Kim SG, Lim SC, Ong JL. Osteogenic activity of the mixture of chitosan and particulate dentin. J Biomed Mater Res A. 2008; 87:618–623. PMID: 18186071.

10. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170. PMID: 7246093.
11. Molly L. Bone density and primary stability in implant therapy. Clin Oral Implants Res. 2006; 17(Suppl 2):124–135. PMID: 16968388.

12. Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis-Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent. 1998; 18:528–543. PMID: 10321168.
13. Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, et al. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999; 14:853–858. PMID: 10612923.
14. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983; (174):28–42. PMID: 6339139.

15. Hatano N, Shimizu Y, Ooya K. Aclinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004; 15:339–345. PMID: 15142097.
16. Kim YK, Kim SG, Lee BG. Bone graft and implant. Vol. 2-2. Seoul: Narae Pub;2007. p. 243–258.
17. Lee JK. Outfracture osteotomy on lateral maxillary wall as a modified sinus graft technique. J Oral Maxillofac Surg. 2010; 68:1639–1641. PMID: 20417015.

18. Song SI, Jeong HR, Kim HM, Lee JK. Clinical investigation on the feasibility of outfracture osteotomy sinus graft technique. J Korean Assoc Oral Maxillofac Surg. 2009; 35:367–371.
20. Kawai T, Urist MR. Bovine tooth-derived bone morphogenetic protein. J Dent Res. 1989; 68:1069–1074. PMID: 2808865.

21. Butler WT, Mikulski A, Urist MR, Bridges G, Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977; 56:228–232. PMID: 265954.

22. Conover MA, Urist MR. Transmembrane bone morphogenesis by implants of dentin matrix. J Dent Res. 1979; 58:1911. PMID: 290656.

23. McKee MD, Aoba T, Moreno EC. Morphology of the enamel organ in the miniature swine. Anat Rec. 1991; 230:97–113. PMID: 2064032.

24. Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000; 407:86–90. PMID: 10993078.

25. Mosekilde L, Weisbrode SE, Safron JA, Stills HF, Jankowsky ML, Ebert DC, et al. Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation. Bone. 1993; 14:379–382. PMID: 8363881.

26. Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011; 11:7442–7445. PMID: 22103215.

Fig. 1
Surgical procedure (lateral window approach) with the outfracture osteotomy sinus graft technique. After extraoral skin incision on the infraorbital area, careful dissection was done until we reached the lateral maxillary sinus wall. After bony window opening, the autogenous tooth-derived material was grafted in the floor of the maxillary sinus. The same procedure was done on the left side of the same animal, except the graft material wherein the synthetic hydroxyapatite was grafted. The same procedure was repeated in the remaining 4 experimental animals on the same day. The area of interest was marked(*) on the floor of the maxillary sinus area.
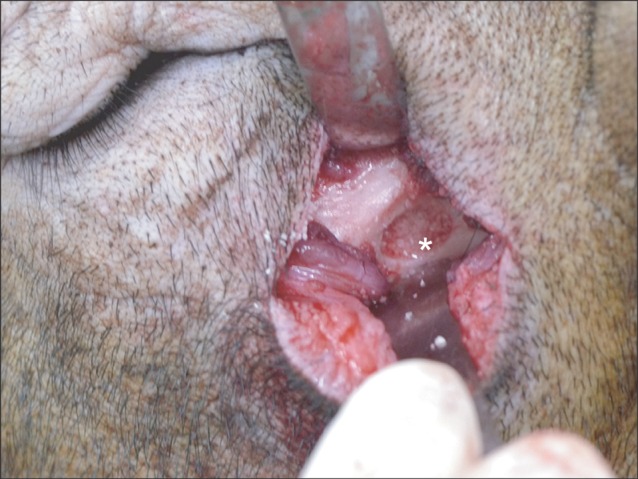
Fig. 2
Typical example of the experimental group at magnification of ×100 (H&E staining). Autogenous tooth-derived graft materials (asterisks) showing multiple linear streaks of the dentinal tubular structure were surrounded by the new bone (arrowheads) growing at the periphery of the graft materials.
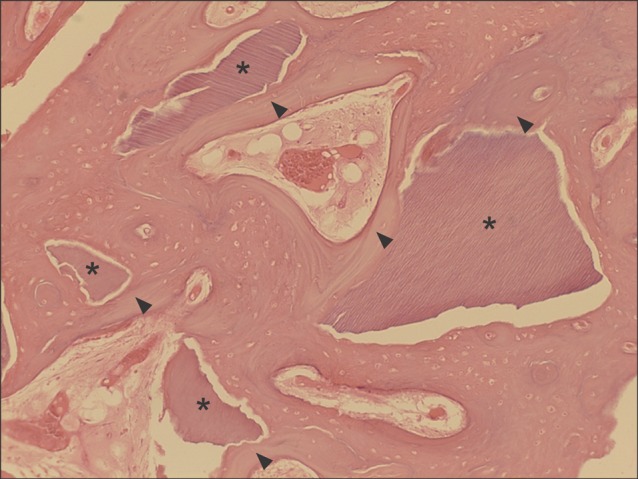
Fig. 3
Another typical example of the experimental group at higher magnification of ×400 (H&E staining). Multinucleated giant cells (arrowhead) were seen at the periphery of the material of multiple linear streaks. This suggests osteoclastic activity at the periphery of the grafted material, which is an evidence of bone remodeling.
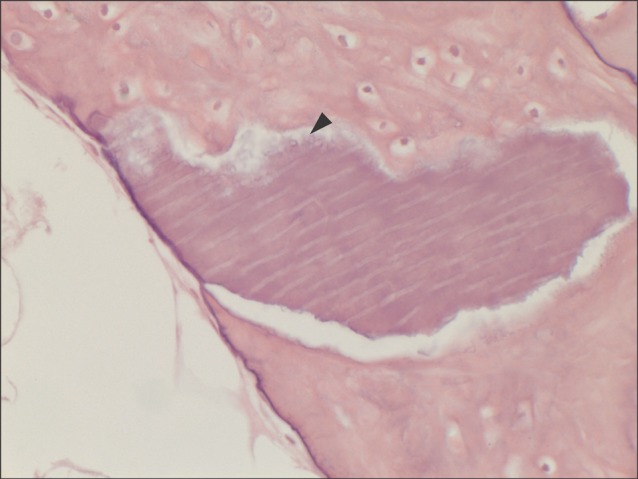
Fig. 4
Another typical example of the experimental group at magnification of ×100 (H&E staining). New bone (arrowhead) was encroaching on the autogenous tooth-derived graft material (asterisk) from the periphery into the center.
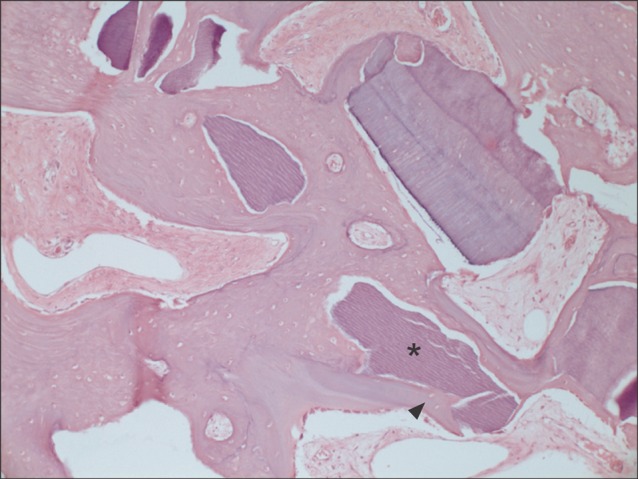
Fig. 5
Typical example of the control group at magnification of ×100 (H&E staining). New bone was also found at the periphery of the grafted hydroxyapatite (arrowheads), but not in the inner portion of the graft space (asterisk).
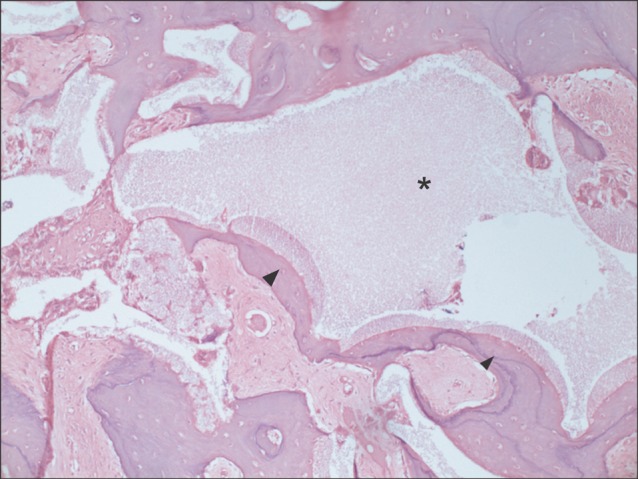
Table 1
Histomorphometric analysis of the new bone 12 weeks after the sinus bone graft
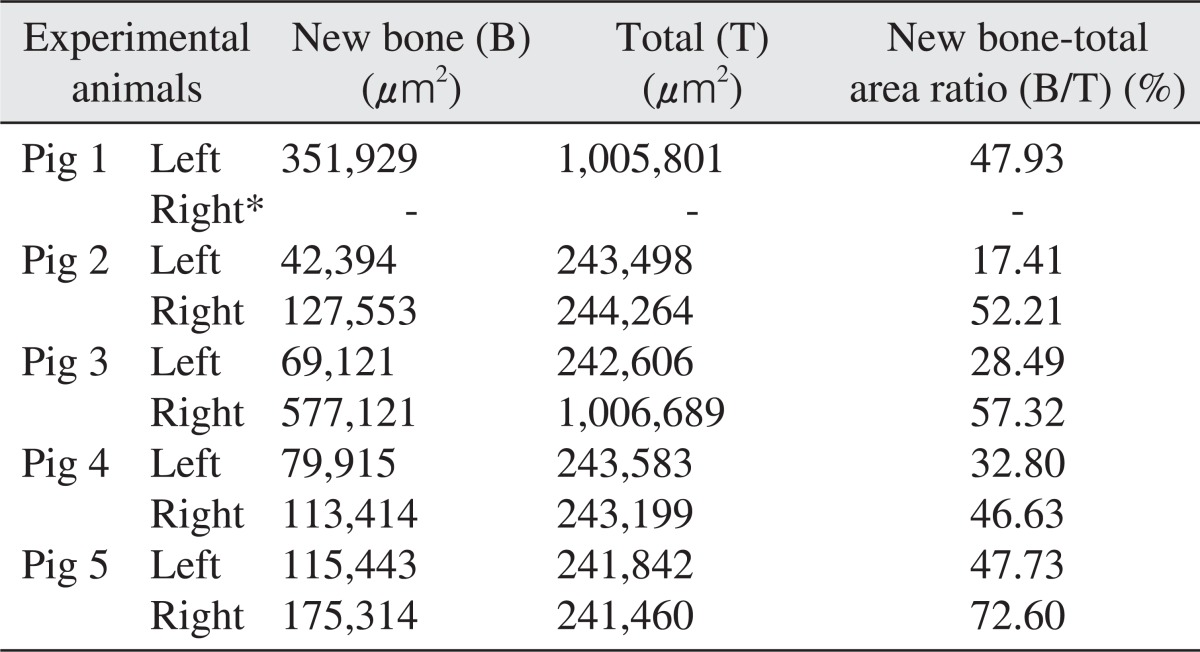
Right sinuses of all experimental pigs were filled with autogenous tooth bone, whereas left sinuses were filled with synthetic hydroxyapatiteas control groups. New bone area (B) and total area (T) was estimated and the ratio of new bone area over total area (B/T) was calculated for each specimen.
*Specimen from the right maxillary sinus (experimental group) of the pig 1 was missing during specimen preparation and was designated as negative in this table.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download